Abstract
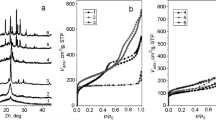
The physicochemical properties of a series of catalysts based on granulated Na-Yh zeolite with the hierarchic (micro–meso–macro) pore structure (Na-Yh, HNa-Yh, MgO/Na-Yh, La2O3/Na-Yh, TiO2/Na-Yh) and the activity and selectivity of these catalysts in isophorone aromatization were studied. MgO/Na-Yh and La2O3/Na-Yh zeolites with high content of basic sites and low content of Brønsted acid sites are the most effective in the synthesis of 3,5-dimethylphenol: The 3,5-dimethylphenol formation selectivity reaches 63–69% at 87–94% isophorone conversion. In the presence of Na-Yh zeolite containing both acid and base sites, the prevalent reactions are isophorone isomerization and synthesis of trimethylbenzenes. The modification of Na-Yh with titanium oxide leads to an increase in the content of acid sites on the TiO2/Na-Yh surface and to the prevalence of the aromatization to form trimethylbenzenes. In the presence of HNa-Yh containing a set of the strongest Brønsted and Lewis acid sites, isophorone transforms into a mixture of polymethylbenzenes.








Similar content being viewed by others
REFERENCES
Bennett, J. and Mabille, C., Advanced Fuel Additives for Modern Internal Combustion Engines, in Alternative Fuels and Advanced Vehicle Technologies for Improved Environmental Performance, Amsterdam: Elsevier; 2022. p. 197–229. https://doi.org/10.1016/B978-0-323-90979-2.00002-0
Bloomfield, S.F. and Miller, E.A., J. Hosp. Infect., 1989, vol. 13, pp. 231−239. https://doi.org/10.1016/0195-6701(89)90003-0
Deng, Q., Nie, G., Pan, L., Zou, J.-J., Zhang, X., and Wang, L., Green Chem., 2015, vol. 17, pp. 4473−4481. https://doi.org/10.1039/C5GC01287B
Raju, D.B., Rao Rama, K.S., Salvapathi, G.S., Prasad Sai, P.S., and Rao, K.P., Appl. Catal. A: General, 2000, vol. 193, pp. 123–128. https://doi.org/10.1016/S0926-860X(99)00418-4
Wang, D., Zhenyu, L., and Qingya, L., Ind. Eng. Chem. Res., 2019, vol. 58, no. 16, pp. 6226–6234. https://doi.org/10.1021/acs.iecr.9b00175
Feng, B., Jing, Y., Guo, Y., Liu, X., and Wang, Y., Green Chem., 2021, vol. 23, pp. 9640–9645. https://doi.org/10.1039/D1GC02767K
Granda, M., Blanco, C., Alvarez, P., Patrick, J.W., and Menéndez, R., Chem. Rev., 2014, vol. 114, no. 3, pp. 1608−1636. https://doi.org/10.1021/cr400256y
Kirichenko, G.N., Glazunova, V.I., Kirichenko, Y.Yu., and Dzhemilev, U.M., Petrol. Chem., 2006, vol. 46, pp. 434–438. https://doi.org/10.1134/S0965544106060090
Patent CN 1583697A, 2005.
Salvapati, G.S., Ramanamurty, K.V., Janardanarao, M., and Vaidyeswaran, R., Appl. Catal. A: General, 1989, vol. 48, pp. 223–233. https://doi.org/10.1016/S0166-9834(00)82794-3
David Raju, B., Rama Rao, K.S., Salvapathi, G.S., Sai Prasad, P.S., and Rao Kanta, P., Appl. Catal. A: General, 2001, vol. 209, pp. 335–344. https://doi.org/10.1016/S0926-860X(00)00771-7
Patent US 4453025, 1984.
Patent CN 108083962A, 2018.
Patent CN 107793297A, 2018.
Kerstens, D., Smeyers, B., Van Waeyenberg, J., Zhang, Q., Yu, J., and Sels, B.F., Adv. Mater., 2020, vol. 32, no. 44, article 2004690. https://doi.org/10.1002/adma.202004690
Chal, R., Gérardin, C., Bulut, M., and van Donk, S., ChemCatChem, 2011, vol. 3, pp. 67–81. https://doi.org/10.1002/cctc.201000158
Serebrennikov, D.V., Grigor’eva, N.G., Khazipova, A.N., Samigullina, Z.S., and Kutepov, B.I., Kinet. Catal., 2022, vol. 63, pp. 577–584. https://doi.org/10.1134/S0023158422050093
Grigor’eva, N.G., Filippova, N.A., Bubennov, S.V., Khazipova, A.N., Kutepov, B.I., and Dyakonov, V.A., Petrol. Chem., 2021, vol. 61, no. 3, pp. 364–369. https://doi.org/10.1134/S0965544121030075
Grigor’eva, N.G., Filippova, N.A., Bubennov, S.V., and Kutepov, B.I., Petrol. Chem., 2022, vol. 62, pp. 942–949. https://doi.org/10.1134/S0965544122070131
Grigorieva, N.G., Bayburtli, A.V., Travkina, O.S., Bubennov, S.V., Kuvatova, R.Z., Arteméva, A.S., and Kutepov, B.I., ChemistrySelect, 2022, vol. 7, no. 11, article e202103532. https://doi.org/10.1002/slct.202103532
Patent RU 2456238, 2012.
Travkina, O.S., Agliullin, M.R., Filippova, N.A., Khazipova, A.N., Danilova, I.G., Grigorʼeva, N.G., Narender, N., Pavlov, M.L., and Kutepov, B.I., RSC Adv., 2017, vol. 7, pp. 32581–32590. https://doi.org/10.1039/C7RA04742H
Pavlov, M.L., Travkina, O.S., Khazipova, A.N., Basimova, R.A., Shavaleeva, N.N., and Kutepov, B.I., Petrol. Chem., 2015, vol. 55, no. 7, pp. 552–556. https://doi.org/10.1134/S0965544115070105
Tamura, M., Shimizu, K., and Satsuma, A., Appl. Catal. A: General, 2012, vols. 433–434, pp. 135–145. https://doi.org/10.1016/j.apcata.2012.05.008
Morterra, C., Chiorino, A., Ghiotti, G., and Garrone, E., J. Chem. Soc., Faraday Trans. 1, 1979, vol. 75, pp. 271–288. https://doi.org/10.1039/F19797500271
Huber, S. and Knözinger, H., J. Mol. Catal. A: Chemical, 1999, vol. 141, pp. 117–127. https://doi.org/10.1016/S1381-1169(98)00255-6
Raju, B.D., Rao, K.S.R., Salvapathi, G.S., Prasad, P.S.S., and Rao, P.K., Top. Catal., 2004, vol. 29, nos. 3–4, pp. 167–174. https://doi.org/10.1023/B:TOCA.0000029799.19387.b7
Faba, L., Díaz, E., and Ordónez, S., Appl. Catal. B: Environmental, 2013, vols. 142–143, pp. 387–395. https://doi.org/10.1016/j.apcatb.2013.05.043
Funding
The study was financially supported by the Russian Science Foundation, project no. 23-13-00213.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Additional information
Publisher's Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Grigor’eva, N.G., Kirsanov, V.Y., Korzhova, L.F. et al. Metal-Containing Granulated Yh Zeolites with Hierarchic Structure in Isophorone Aromatization. Pet. Chem. (2024). https://doi.org/10.1134/S0965544124020038
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S0965544124020038