Abstract
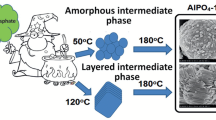
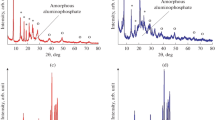
Crystallization of aluminophosphate gels prepared using boehmite and phosphoric acid with different di-n-propylamine/Al2O3 ratios was studied. Depending on the di-n-propylamine/Al2O3 ratio, the following intermediate phases can be formed in reaction gels in the course of further crystallization: hydrated aluminophosphate AlPO4·2H2O, the layered phase, and their mixtures. Procedures were suggested for controlling the morphology of the AlPO4-11 molecular sieve, based on variation of the template/Al2O3 ratio in the course of the reaction gel preparation.










Similar content being viewed by others
REFERENCES
Vermeiren, W. and Gilson, J.P., Top. Catal., 2009, vol. 52, pp. 1131–1161. https://doi.org/10.1007/s11244-009-9271-8
Hartmann, M. and Elangovan, S., Adv. Nanopor. Mater., 2010, vol. 1, pp. 237–312. https://doi.org/10.1016/S1878-7959(09)00104-2
Potter, M.E., ACS Catal., 2020, vol. 10, pp. 9758–9789. https://doi.org/10.1021/acscatal.0c02278
Baerlocher, C., McCusker, L.B., and Olson, D.H., Atlas of Zeolite Framework Types, Amsterdam: Elsevier, 2007, 6th ed., p. 404.
Raja, R., Sankar, G., and Thomas, J.M., J. Am. Chem. Soc., 2001, vol. 123, no. 33, pp. 8153–8154. https://doi.org/10.1021/ja011001+
Zong, Z., Elsaidi, S.K., Thallapally, P.K., and Carreon, M.A., Ind. Eng. Chem. Res., 2017, vol. 56, pp. 4113–4118. https://doi.org/10.1021/acs.iecr.7b00853
Wang, N., Tang, Z.K., Li, G.D., and Chen, J.S., Nature, 2000, vol. 408, pp. 50–51. https://doi.org/10.1038/35040702
Hu, J., Wang, D., Guo, W., Du, S., and Tang, Z.K., J. Phys. Chem. С, 2012, vol. 116, pp. 4423–4430. https://doi.org/10.1021/jp210451q
Yadav, R. and Sakthivel, A., Appl. Catal. A: General, 2014, vol. 481, pp. 143–160. https://doi.org/10.1016/j.apcata.2014.05.010
Singh, P.S., Bandyopadhyay, R., Hegde, S.G., and Rao, B.S., Appl. Catal. A: General, 1996, vol. 136, no. 2, pp. 249–263. https://doi.org/10.1016/0926-860X(95)00303-7
Nieminen, V., Kumar, N., Heikkilä, T., Laine, E., Villegas, J., Salmi, T., and Murzin, D.Y., Appl. Catal. A: General, 2004, vol. 259, no. 2, pp. 227–234. https://doi.org/10.1016/j.apcata.2003.09.038
Zhu, Z., Chen, Q., Xie, Z., Yang, W., and Li, C., Micropor. Mesopor. Mater., 2006, vol. 88, nos. 1–3, pp. 16–21. https://doi.org/10.1016/j.micromeso.2005.08.021
Wang, X., Guo, F., Wei, X., Liu, Z., Zhang, W., Guo, S., and Zhao, L., Korean J. Chem. Eng., 2016, vol. 33, pp. 2034–2041. https://doi.org/10.1007/s11814-016-0065-y
Agliullin, M.R., Fayzullin, A.V., Fayzullina, Z.R., and Kutepov, B.I., Crystals, 2023, vol. 13, p. 227. https://doi.org/10.3390/cryst13020227
Huang, Y., Demko, B.A., and Kirby, C.W., Chem. Mater., 2003, vol. 15, no. 12, pp. 2437–2444. https://doi.org/10.1021/cm021728c
Albuquerque, A., Coluccia, S., Marchese, L., and Pastore, H.O., Stud. Surf. Sci. Catal., 2004, vol. 154, pp. 966–970. https://doi.org/10.1016/S0167-2991(04)80911-X
Vistad, Ø.B., Akporiaye, D.E., and Lillerud, K.P., J. Phys. Chem. B, 2001, vol. 105, no. 50, pp. 12437–12447. https://doi.org/10.1021/jp0110758
Venkatathri, N., Hegde, S.G., Ramaswamy, V., and Sivasanker, S., Micropor. Mesopor. Mater., 1998, vol. 23, nos. 5–6, pp. 277–285. https://doi.org/10.1016/S1387-1811(98)00123-1
Agliullin, M.R., Serebrennikov, D.V., Khalilov, L., Fayzullina, Z.R., Pavlova, I.N., and Kutepov, B.I., Kinet. Catal., 2023, vol. 64, no. 3, pp. 311–319. https://doi.org/10.1134/S0023158423030011
Agliullin, M.R., Cherepanova, S.V., Kuvatova, R.Z., Faizullin, A.V., Khalilov, L.M., and Kutepov, B.I., Petrol. Chem., 2023, vol. 63, no. 2, pp. 149–157. https://doi.org/10.1134/S0965544123020044
Zhang, B., Xu, J., Fan, F., Guo, Q., Tong, X., Yan, W., Yu, J., Deng, F., Li, C., and Xu, R., Micropor. Mesopor. Mater., 2012, vol. 147, no. 1, pp. 212–221. https://doi.org/10.1016/j.micromeso.2011.06.018
Chen, B. and Huang, Y., J. Phys. Chem. C, 2007, vol. 111, no. 42, pp. 15236–15243. https://doi.org/10.1021/jp071868f
Xu, R., Zhang, W., Xu, J., Tian, Z., Deng, F., Han, X., and Bao, X., J. Phys. Chem. C, 2013, vol. 117, no. 11, pp. 5848–5854. https://doi.org/10.1021/jp400422z
Agliullin, M.R., Kutepov, B.I., Ostroumova, V.A., and Maximov, A.L., Petrol. Chem., 2021, vol. 61, no. 8, pp. 836–851. https://doi.org/10.1134/S0965544121080028
Fan, F., Feng, Z., Sun, K., Guo, M., Guo, Q., Song, Y., Li, W., and Li, C., Angew. Chem., 2009, vol. 121, no. 46, pp. 8899–8903. https://doi.org/10.1002/ange.200903601
Holmes, A.J., Kirkby, S.J., Ozin, G.A., and Young, D., J. Phys. Chem., 1994, vol. 98, no. 17, pp. 4677–4682. https://doi.org/10.1021/j100068a032
Database of Zeolite Structures. http://www.iza-structure.org/databases/
Funding
The study was supported by the Russian Science Foundation, project no. 23-73-00119, https://rscf.ru/project/23-73-00119/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Additional information
Publisher’s Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Agliullin, M.R., Kuvatova, R.Z., Gus’kov, V.Y. et al. Influence of the Template/Al2O3 Ratio in Reaction Aluminophosphate Gels on the Characteristics of Intermediate Phases and AlPO4-11 Molecular Sieves. Pet. Chem. 63, 708–717 (2023). https://doi.org/10.1134/S0965544123060026
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544123060026