Abstract
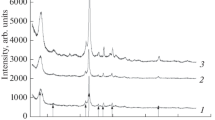
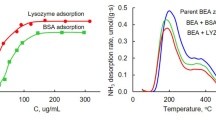
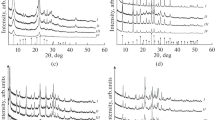
The study investigates the physicochemical, porous/textural, and adsorptive properties, as well as the biological activity of BEA, RHO, PAU, and FAU(Y) synthetic zeolites. The adsorption capacity of the zeolites for oxytocin, lysozyme, and albumin—markers of low- and medium-molecular-weight pathological proteins—was measured. The concentration-dependent effects of the zeolites on the viability of human endothelial cells (Ea.hy926) were evaluated. At concentrations of 2.5 to 10 mg/mL, the Y zeolite was found to exhibit no pronounced cytotoxicity. In the presence of the BEA, the cell viability decreased to 80% even at 2.5 mg/mL. The RHO and PAU were shown to have the highest cytotoxicity and significantly inhibit the growth of human cells (down to 23–35%). The study demonstrated that only BEA and Y, among the synthetic zeolites tested, have good biomedical potential due to their high adsorption capacity, low hemolytic activity, and low cytotoxicity.






Similar content being viewed by others
Notes
The hemolytic activity is the ability to induce hemolysis, i.e. to destroy red blood cells, leading to the release of hemoglobin into the cell environment.
MTT assay is a colorimetric assay for assessing cell metabolic activity.
REFERENCES
Ronco, C. and Bellomo, R., Crit. Care, 2022, vol. 26, no. 135, pp. 1–12. https://doi.org/10.1186/s13054-022-04009-w
Laurino, C. and Palmieri, B., Nutricion Hospitalaria, 2015, vol. 32, no. 2, pp. 573–581. https://doi.org/10.3305/nh.2015.32.2.8914
Clark W., R., Ferrari, F., La Manna, G., and Ronco, C., Contribut. Nephrol., 2017, pp. 43–57. https://doi.org/10.1159/000468911
Panichev, A.M., Bogomolov, N.I., Bogatova, N.P., Silkin, S.N., and Gulkov, A.N., Zeolites in Surgery, Vladivostok: FESTU, 2004, p. 120.
Souza, G., Villén, I., Viseras, F., and Perger Sibele, C., Pharmaceutics, 2023, no. 15(1352), pp. 1–33. https://doi.org/10.3390/pharmaceutics15051352
Norouzian, M.A., Valizadeh, R., Khadem, A.A., Afzalzadeh, A., and Nabipour, A., Biol. Trace Element Res., 2010, vol. 137, no. 2, pp. 168–176. https://doi.org/10.1007/S12011-009-8574-8
Tomeckova, V., Rehakova, M., Mojzisova, G., Wadsten, T., Zelenakova, K., and Komanicky, V., Spectroscop. Lett., 2015, vol. 49, no. 2, pp. 63–72. https://doi.org/10.18097/PBMC20226803201
Amorim, R., Vilaca, N., Martinho, O., Reis, R.M., Sardo, M., Rocha, J., Fonseca, A.M., Baltazar, F., and Neves, I.C., J. Phys. Chem. C, 2012, vol. 116, no. 48, pp. 25642–25650. https://doi.org/10.1021/jp3093868
Berry, T.A., Belluso, E., Vigliaturo, R., Gieré, R., Emmett, E.A., Testa, J.R., Steinhorn, G., and Wallis, S.L., Int. J. Environ. Res. Publ. Health, 2022, vol. 19, no. 4031, pp. 1–17. https://doi.org/10.3390/ijerph19074031
Zhang, H., Kim, Ya., and Dutta, P.K., Micropor. Mesopor. Mater., 2006, vol. 88, nos. 1–3, pp. 312–318. https://doi.org/10.1016/J.MICROMESO.2005.09.026
Tang, T., Zhang, L., Dong, H., Fang, Z., Yu, Q., and Tang, T., RSC Adv., 2017, vol. 7, pp. 7711–7717. https://doi.org/10.1039/C6RA27129D
Golubeva, O.Yu. and Ul’yanova, N.Yu., Glass Phys. Chem., 2015, vol. 41, no. 5, pp. 537–544. https://doi.org/10.1134/S1087659615050065
Peters, T., Adv. Protein Chem., 1985, vol. 37, pp. 161–245. https://doi.org/10.1016/s0065-3233(08)60065-0
Ganz, T., Encycloped. Respirat. Med., 2006, pp. 649–653. https://doi.org/10.1016/b0-12-370879-6/00228-3
Henschen, A., Hupe, K.-P., Lottspeich, F., and Voelter, W., The Quarterly Rev. Biol., 1986, vol. 61, no. 4, pp. 532–536. https://doi.org/10.1086/415159
Tas, A.C., Biomaterial., 2000, vol. 21, no. 14, pp. 1429–1438. https://doi.org/
Antibacterial Peptide Protocols, Shafer, W.M., Ed., Humana Press., 1997, vol. 78, pp. 255–257.
Ulyanova, N.Yu., Kurylenko, L.N., Shamova, O.V., Orlov, D.S., and Golubeva, O.Yu., Glass Phys. Chem., 2020, vol. 46, pp. 155–161. https://doi.org/10.1134/S108765962002011X
Golubeva, O., Ulyanova, N., Yakovlev, A.V., Glass Phys. Chem., 2015, vol. 41, pp. 413–416. https://doi.org/10.1134/S1087659615040069
Funding
This study was carried out within the State Program of ISC RAS (project no. 0081-2022-0001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest requiring disclosure in this article.
Additional information
Publisher’s Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ul’yanova, N.Y., Brazovskaya, E.Y., Golubeva, O.Y. et al. Adsorption Capacity and Biological Activity of Synthetic Zeolites. Pet. Chem. 63, 790–797 (2023). https://doi.org/10.1134/S096554412305002X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S096554412305002X