Abstract
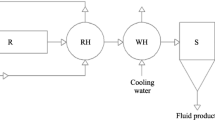
This work is devoted to the mathematical modeling and calculation of the hydrogenation reaction of carbon dioxide to produce methanol using kinetic models developed with taking into account different understandings of the reaction mechanism; in particular, the Graaf and Rozovskii–Lin mechanisms are considered. It has been shown that under flow conditions, both models designed for describing the reaction of methanol production from synthesis gas fairly accurately describe the CO2 hydrogenation reaction. Under recycle flow conditions, a more accurate description of the product yield with changes in pressure and temperature can be obtained using a model based on the Graaff mechanism.



Similar content being viewed by others
REFERENCES
A. S. Kosoi, Yu. A. Zeigarnik, O. S. Popel’, et al., Teploenergetika, No. 9, 23 (2018).
A. Álvarez, A. Bansode, A. Urakawa, et al., Chem. Rev. 117, 9804 (2017).
M. Bowker, Chem. Cat. Chem. 11, 4238 (2019).
I. U. Din, M. S. Shaharun, M. A. Alotaibi, et al., J. CO2 Util. 34, 20 (2019).
Sh. Dang, H. Yang, P. Gao, et al., Catal. Today 330, 61 (2019).
R. Guil-López, N. Mota, J. Llorente, et al., Materials 12, 3902 (2019).
S. K. Wilkinson, L. G. A. van de Water, B. Miller, et al., J. Catal. 337, 208 (2016).
J.-F. Portha, K. Parkhomenko, K. Kobl, et al., Ind. Eng. Chem. Res. 56, 13 133 (2017).
A. A. Kiss, J. J. Pragt, H. J. Vos, et al., Chem. Eng. J. 284, 260 (2016).
G. Bozzano and F. Manenti, Prog. Energy Combust. Sci. 56, 71 (2016).
T. Kubota, I. Hayakawa, H. Mabuse, et al., Appl. Organomet. Chem. 15, 121 (2001).
G. H. Graaf, E. J. Stamhuis, and A. A. C. M. Keenackers, Chem. Eng. Sci. 43, 3185 (1988).
A. Y. Rozovskii and G. I. Lin, Top. Catal. 22, 137 (2003).
https://docs.scipy.org/doc/scipy/reference/tutorial/optimize.html. Accessed July 10, 2020.
https://docs.scipy.org/doc/scipy/reference/generated/scipy.integrate.odeint.html. Accessed July 10, 2020.
G. I. Lin, P. V. Samokhin, and M. A. Kipnis, Catal. Ind. 12, 101 (2020).
H. Renon and J. M. Prausnitz, AIChE J. 14, 135 (1968).
Funding
The work was supported by the Russian Science Foundation, grant no. 17-73-30046.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest requiring disclosure in this article.
Additional information
Translated by S. Zatonsky
Rights and permissions
About this article
Cite this article
Magomedova, M.V., Starozhitskaya, A.V., Afokin, M.I. et al. Mathematical Modeling and Calculation of the Methanol Production Process via Carbon Dioxide Hydrogenation. Pet. Chem. 60, 1244–1250 (2020). https://doi.org/10.1134/S0965544120110146
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544120110146