Abstract
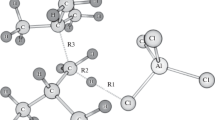
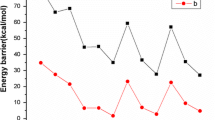
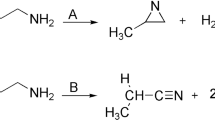
The mechanism of interaction of adamantane with propylene has been studied by quantum-chemical calculations within the density functional theory (DFT). It has been shown that the main products of adamantane alkylation with propylene in the presence of acid catalysts are hydrocarbons with unbranched (normal-chain) substituents, 1-n-propyl- and 1-n-propenyladamantanes. The main stages of adamantane alkylation and the geometric and electronic structures of the intermediates have been determined. The thermodynamic characteristics of the studied adamantanes have been found, and the mechanisms of individual steps of their transformations have been proposed. The obtained data make it possible to control the process of preparation of substituted adamantanes with a defined structure that are of interest for the development of efficient energy-rich materials, high-density fuels, and thermally stable polymers.








Similar content being viewed by others
REFERENCES
S. Landa and V. Machacek, Coll. Czech. Chem. Commun. 5, 1 (1933). https://doi.org/10.1135/cccc19330001
E. I. Bagrii, Adamantanes: Preparation, Properties, and Use (Nauka, Moscow, 1989) [in Russian].
M. A. Gunawan, J. C. Hierso, D. Poinsot, et al., New J. Chem. 38, 28 (2014).https://doi.org/10.1039/C3NJ00535F
A. I. Nekhaev, E. I. Bagrii, and A. L. Maksimov, Pet. Chem. 51, 86 (2011). https://doi.org/10.1134/S0965544111020095
I. A. Novakov, B. S. Orlinson, Z. M. Sabirov, et al., Vysokomol. Soedin., Ser. B 35, 2053 (1993).
A. S. Barnard and M. Sternberg, J. Mater. Chem. 17, 4811 (2007). https://doi.org/10.1039/b710189a
H. Wu, H. Xu, F. Tao, et al., New J. Chem. 42, 12 802 (2018). https://doi.org/10.1039/c8nj01881b
A. Kovalenko, C. Yumusak, P. Heinrichova, et al., J. Mater. Chem. C 5, 4716 (2017). https://doi.org/10.1039/C6TC05076J
K. Katsumasa and O. Susumu, Jpn. J. Appl. Phys. 51, 015 001 (2012). https://doi.org/10.1143/JJAP.51.015001
A. Datta, M. Kirca, Y. Fu, and A. C. To, Nanotechnology 22, 065 706 (2011). https://doi.org/10.1088/0957-4484/22/6/065706
Yu. A. Borisov and E. I. Bagrii, Dokl. Phys. Chem. 463, 141 (2015). https://doi.org/10.1134/S0012501615070015
E. I. Bagrii, Yu. A. Borisov, Yu. A. Kolbanovskii, and A. L. Maksimov, Pet. Chem. 59, 66 (2019). https://doi.org/10.1134/S0965544119010067
E. A. Zauer and O. A. Zauer, Russ. J. Phys. Chem. A 83, 582 (2009). https://doi.org/10.1134/S0036024409040128
R. Abbasoğlu and S. S. Yılmaz, J. Mol. Struct. (THEOCHEM) 589, 431 (2002). https://doi.org/10.1016/S0166-1280(02)00278-6
J. O. Jensen, Spectrochim. Acta, Part A 60, 1895 (2004). https://doi.org/10.1016/j.saa.2003.09.024
S. A. Kovács and A. Szabó, J. Mol. Struct. 519, 13 (2000). https://doi.org/10.1016/S0022-2860(99)00278-1
W. D. S. A. Miranda, S. S. Coutinho, M. S. Tavares, et al., J. Mol. Struct. 1122, 299 (2016). doi . 2016.05.103https://doi.org/10.1016/j.molstruc
S. Sauer, I. Paidarová, P. Čársky, and R. Čurík, Eur. Phys. J. D 70, 105 (2016). https://doi.org/10.1140/epjd/e2016-70084-x
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, et al., J. Comput. Chem. 14, 1347 (1993). https://doi.org/10.1002/jcc.540141112
Chemcraft. www.chemcraftprog.com. Accessed April 25, 2019.
Funding
This publication was supported by the “5-100” Program of the Peoples’ Friendship University of Russia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR INFORMATION
N.I. Baranov, ORCID: https://orcid.org/0000-0002-8813-7786
R.E. Safir, ORCID: https://orcid.org/0000-0002-1981-0594
E.I. Bagrii, ORCID: https://orcid.org/0000-0002-9652-9296
K.V. Bozhenko, ORCID: https://orcid.org/0000-0002-5786-5297
A.G. Cherednichenko, ORCID: https://orcid.org/0000-0002-4709-5313
Additional information
Translated by E. Boltukhina
Rights and permissions
About this article
Cite this article
Baranov, N.I., Safir, R.E., Bagrii, E.I. et al. Catalytic Alkylation of Adamantane with Propylene: Quantum-Chemical Calculations and Experimental Data. Pet. Chem. 60, 1033–1042 (2020). https://doi.org/10.1134/S0965544120090042
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544120090042