Abstract
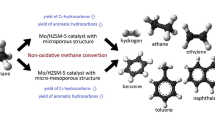
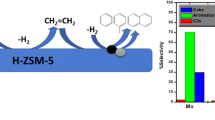
The nonoxidative conversion of methane to aromatic hydrocarbons in the presence of a high-silica ZSM-5 zeolite modified with molybdenum and rhenium nanopowders has been studied. Data on the acid characteristics of the catalysts have been derived by temperature-programmed desorption of ammonia. The microstructure and composition of the Re/ZSM-5 and Re–Mo/ZSM-5 catalyst systems have been studied by transmission electron microscopy. It has been shown that modification of a Mo-containing zeolite with rhenium leads to an increase in the activity and stability of the catalyst in the methane dehydroaromatization reaction.







Similar content being viewed by others
REFERENCES
Y. Xu and L. Lin, Appl. Catal., A 188, 53 (1999).
L. Wang, R. Ohnishi, and M. Ichikawa, J. Catal. 190, 276 (2000).
A. Szoke and F. Solymosi, Appl. Catal., A 142, 361 (1996).
R. Kojima, S. Kikuchi, H. T. Ma, et al., Catal. Lett. 110, 15 (2006).
V. Abdelsayed, D. Shekhawat, and M. W. Smith, Fuel 139, 401 (2015).
S. Maihi, P. Mohanty, H. Wang, and K. K. Pant, J. Energy Chem. 22, 543 (2013).
V. I. Bukhtiyarov, V. I. Zaikovskii, A. S. Kashin, and V. P. Ananikov, Usp. Khim. 85, 1198 (2016).
R. Ohnishi and M. Ichikawa, Catal. Surv. Jpn. 5, 103 (2002).
M. A. Ryashentseva and Kh. M. Minachev, Usp. Khim. 38, 2050 (1969).
F. Solymosi, J. Cserenyi, A. Szoke, et al., J. Catal. 165, 150 (1997).
D. Wang, J. H. Lunsford, and M. P. Rosynek, J. Catal. 169, 347 (1997).
S. Liu, L. Wang, R. Ohnishi, and M. Ichikawa, J. Catal. 181, 175 (1999).
R. W. Borry, Y. H. Kim, A. Huffsmith, et al., J. Phys. Chem. B 103, 5787 (1999).
Y. Xu, Y. Shu, S. Liu, et al., Catal. Lett. 35, 233 (1995).
L. Wang, R. Ohnishi, and M. Ichikawa, J. Catal. 190, 276 (2000).
V. I. Zaikovskii, A. V. Vosmerikov, V. F. Anufrienko, et al., Kinet. Catal. 47, 389 (2006).
ACKNOWLEDGMENTS
This work was performed under the Basic Research Program of State Academies of Sciences, project no. V.46.2.1.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by M. Timoshinina
Rights and permissions
About this article
Cite this article
Stepanov, A.A., Zaikovskii, V.I., Korobitsyna, L.L. et al. Nonoxidative Conversion of Methane to Aromatic Hydrocarbons in the Presence of ZSM-5 Zeolites Modified with Molybdenum and Rhenium. Pet. Chem. 59, 91–98 (2019). https://doi.org/10.1134/S0965544119010146
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0965544119010146