Abstract
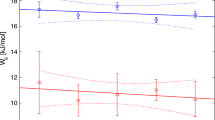
Data on Na and K distribution between feldspar and solution at 550°C and 1.5 kbar under hydrothermal conditions were obtained in experiments on cation-exchange equilibria of gallium feldspars according to the reaction NaGaSi3O8 + KCl aq = KGaSi3O8 + NaCl aq. The distribution of Na and K between feldspars and fluid is nonideal: in the sodium part of the system, potassium enriches the solution relative to feldspar, then an inversion occurs and, at \(X_{{\text{K}}}^{{Fsp}}\) > 0.8, potassium is redistributed into feldspar relative to the fluid. The existence of a region of immiscibility of the solid solution is shown, the concentration dependences of the Na and K distribution coefficients between feldspar and fluid are determined, and the unit cell parameters of the (Na,K)GaSi3O8 solid solutions were refined. The excess volume of mixing of solid solutions of gallium alkali feldspars is estimated from X-ray diffraction (XRD) data. Correlations between the excess integral energies and mixing volumes with structural parameters were identified. The derived dependences make it possible to derive the values of the energy functions of mixing of solid solutions of framework aluminosilicates from structural parameters.






Similar content being viewed by others
REFERENCES
Bambauer, H.U., Kroll, H., Nager, H.E., and Pentinghaus, H., Feldspat-mischkristalle - eine ubersicht, Bull. Soc. Fr. Mineral. Cristallogr., 1974, vol. 97, pp. 313–345.
Bambauer, H.U., Schops, M., and Pentinghaus, H., Feldspar phase relations in the system NaAlSi3O8–SrAl2Si2O8, Bull. Mineral., 1984, vol. 107, pp. 541–551.
Bodnar, R.J., Burnham, C.W., and Sterner, M.S., Synthetic fluid inclusions in natural quartz. iii. determination of phase equilibrium properties in the system H2O–NaCl to 1000°C and 1500 bars, Geochim. Cosmochim. Acta, 1985, vol. 49, pp. 1861–1873.
Burnham, C.W., Least-squares Refinement of Crystallographic Lattice Parameters for IBM PC/XT/AT and Compatibles, Cambridge: Harvard University, 1991.
Carron, J.P. and Lagache, M., Étude expérimentale du fractionnement des éléments Rb, Cs, Sr, et Ba, entre les feldspaths alcalins, solutions hydrothermales et liquides silicatés dans le système Q.Ab.Or.H2O à 2 kbar entre 700 et 800°C, Bull. Minéral., 1980, vol. 103, pp. 571–578.
Chichagov, A.V., Information-calculating system on crystal structure data of minerals (myncryst), Mater. Sci. Forum. Trans Tech Publications. Switzerland, 1994, vol. 166–169, pp. 187–192.
Deckers, B., Kroll, H., and Pentinghaus, H., Mechanism and kinetics of Na,K-unmixing in Al(Si,Ge) alkali feldspars, Mater. Sci. For., 1986, vol. 7, pp. 103–112.
Fleet, M.E., Structures of low gallium albite (NaGaSi3O8) and intermediate germanium albite (NaAlGe3O8): tetrahedral-site ordering in sodium feldspar, Am. Mineral., 1992, vol. 77, pp. 76–84.
Kimata, M., Saito, S., and Shimizu, M., Structure of sanidine type KGaSi3O8 tetrahedral-site disordering in potassium feldspar, Eur. J. Mineral., 1995, vol. 7, pp. 287–296.
Kotel’nikov, A.R., Calculation of mixing functions of plagioclase solid solution, Geokhimiya, 1980, no. 2, pp. 226–230.
Kotel’nikov, A.R., Isomorphism in Framework Silicates, Extended Abstract of Doctoral (Geol.-Min) Dissertation, Moscow: MGU, 1995.
Kotel’nikov, A.R., Kravchuk, I.F., Romanenko, I.M., and Karabtsov, A.A., Experimental study of Na and Sr partitioning between plagioclase and aqueous–salt fluid at 800oC and 2 kbar, Geokhimiya, 1987, no. 1, pp. 33–43.
Kotel’nikov, A.R., Chernysheva, I.V., Romanenko, I.M., and Tikhomirova, V.I., Experimental determination of mixing energy of the Ca–Sr anorthites: cation-exchange equilibrium data, Geokhimiya, 1989, no. 11, pp. 1575–1585.
Kotel’nikov, A.R., Romanenko, I.M., and Kotel’nikova, Z.A., Experimental study of barium partitioning between plagioclase series NaAlSi3O8–BaAl2Si2O8 and aqueous–salt fluid at 800°C and 2 kbar, Geokhimiya, 1990, no. 3, pp. 346–355.
Kotel’nikov, A.R., Bychkov A.M., Zyryanov, V.N., et al., Phase transformation of zeolite and feldspar as a way to create aluminosilicate matrix for radionuclide binding, Geokhimiya, 1995, no. 10, pp. 1527–1532.
Kotel’nikov, A.R., Minerals as matrix material for radionuclide fixation, Geoekologiya, 1997, vol. 6, pp. 3–15.
Kotel’nikov, A.R., Chernysheva, I.V., Kotel’nikova, Z.A., and Senin, V.G., Experimental study of isomorphism in K–Ba feldspars, Geochem. Int., 1999, vol. 37, no. 4, pp. 340–349.
Kotel’nikova, Z.A., Ivanov, D.Yu., and Kotel’nikov, A.R., Phase state of high-temperature chloride solutions of alkaline and alkali-earth metals: data on synthetic fluid inclusions, Tr. IX Mezhdunarodnoi konferentsii po termobarogeokhimii (Proc. 9th Inernational Conference on Thermobarogeochemistry), Aleksandrov: VNIIISIMS, 1999, pp. 252–262.
Kotelnikov, A.R., Schipalkina, N.V., and Suk, N.I., Synthesis of As-containing feldspars and feldspathoids, Exp. GeoSci., 2019, vol. 25, no. 1, pp. 99–102.
Kroll, H., Schmiemann, I., and von Colln, G., Feldspar solid solutions, Am. Mineral., 1986, vol. 71, pp. 1–16.
Kroll, H., Kotelnikov, A.R., Goettlisher, J., and Valyashko, T.V., (K,Sr)-feldspar solid solutions: the volume behavior of heterovalent feldspars, Eur. J. Mineral., 1995, vol. 7, pp. 489–499.
Lagache, M., Etude experimentale du partage de k et ca entre sanidine–anortite et solutions hydrothermales: influence de la temperature, Bull. Mineral, 1988, vol. 111, pp. 471–475.
Orville, P.M., Alkali ion exchange between vapor and feldspar phases, Am. J. Sci., 1963, vol. 261, pp. 201–237.
Orville, P.M., Plagioclase cation exchange equilibria with aqueous chloride solution: results at 700°c and 2000 bars, Am. J. Sci., 1972, vol. 272, no. 3, pp. 234–273.
Pentinghaus, H., Polimorphie in den feldspatbildenden systemen A+T3+T4+O8 und A2+T23+T24+O8 Alkali- und Erdalkali-, Bor-, Aluminium-, Gallium-, Eisen-Silicate und -Germanate, Habil. Diss. Munster, 1980.
Perchuk, L.L. and Ryabchikov, I.D., Fazovoe sootvetstvie v mineral’nykh sistemakh (Phase Correspondence in the Mineral Systems), Moscow: Nedra, 1976.
Phillips, M.V. and Ribbe, P.H., The structures of monoclinic potassium-rich feldspars, Am. Mineral., 1973, vol. 58, pp. 263–270.
Pichavant, M., Schnapper, D., and Brown, W.L., Al-substitution in alkali feldspars: preliminary hydrothermal data in the system NaAlSi3O8–NaBSi3O8, Bull. Mineral., 1984, vol. 107, pp. 529–537.
Ravich, M.I., Vodno-solevye sistemy pri povyshennykh temperaturakh i davleniyakh (Aqueous–Salt Systems at Elevated Pressures and Temperatures), Moscow: Nauka, 1974.
Reed, S.J.B., Electron Microprobe Analysis and Scanning Electron Microscopy in Geology, Cambridge: Cambridge University Press, 2005.
Ribbe, P.H. and Gibbs, G.V., Statistical analysis and discussion of mean Al/Si–O bond distances and the aluminium content of tetrahedral in feldspars, Am. Mineral., 1969, vol. 54, pp. 85–96.
Saxena, S., Thermodynamics of Rock-Forming Crystalline Solutions, Berlin: Heidelberg, 1973.
Shannon, R.P. and Prewitt, C.T., Revised values of effective ionic radii, Acta Crystallogr., 1970, vol. 26, pp. 1046–1048.
Shvedenkov, G.Yu. and Shvedenkova, S.V., Polevye shpaty pod davleniem vody i dvuokisi ugleroda (Feldspars under Water and Carbon Dioxide Pressure), Novosibirsk SO: Nauka, 1982.
Smith, J.V., Feldspar minerals, New York: Springer, 1974.
Sterner, S.M. and Chou, I.-Ming, Downs, R.T., Pitzer Kenneth, S., Phase relations in the system NaCl–KCl–H2O: V. Thermodynamic-ptx analysis of solid-liquid equilibria at high temperatures and pressures, Geochim. Cosmochim. Acta, 1992, vol. 56, no. 6, pp. 2295–2309.
Valyashko, V.M., Fazovye ravnovesiya i svoistva gidrotermal’nykh sistem (Phase Equilibria and Properties of Hydrothermal Systems), Moscow: Nauka, 1990.
Vasil’ev, N.S., Stability and Kinetics of Structural Transformations of Alkali Feldspars, Extended Abstract of Candidate’s (Chem.) Dissertation, Moscow: GEOKhI AN SSSR, 1990.
Zyryanov, V.N., Fazovoe sootvetstvie v sistemakh shchelochnykh polevykh shpatov i fel’dshpatoidov (Phase Correspondence in Alkali Feldspar and Feldspathoid Systems). Moscow: Nauka, 1981.
Funding
This study was carried out under government-funded research project AAAA-A18-118020590151-3 for the Korzhinskii Institute of Experimental Mineralogy, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by E. Kurdyukov
Rights and permissions
About this article
Cite this article
Kotelnikov, A.R., Suk, N.I., Akhmedzhanova, G.M. et al. Experimental Study of Cation-Exchange Equilibria of Solid Solutions of Gallium Feldspars (Na,K)GaSi3O8 with Water–Salt Fluid (NaCl–KCl–H2O) at 550°С and 1.5 Kbar. Petrology 29, 561–574 (2021). https://doi.org/10.1134/S0869591121050039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0869591121050039