Abstract
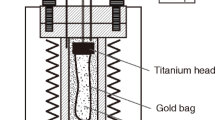
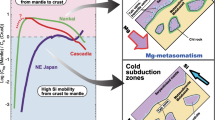
This paper reports the results of high-pressure experimental modeling of interaction between glaucophane schist and harzburgite or websterite for the evaluation of the influence of mantle material on the input–output of components and character of metasomatic transformations at the crust–mantle boundary in the subduction zone. In all experiments, glaucophane schist (proxy for oceanic crust) containing volatile components (H2O and CO2) incorporated in hydrous minerals (amphiboles, phengite, and epidote) and calcite was loaded into the bottom of each capsule and overlain by mantle material. During the experiments at a temperature of 800°C and a pressure of 2.9 GPa, which correspond to the conditions of a hot subduction zone, the schist underwent partial (up to 10%) eclogitization with the formation of the anhydrous assemblage omphacite + garnet + quartz ± magnesite ± potassic phase. Carbonate and a potassic phase were formed only in the experiments with websterite in the upper layer. A reaction zone was formed at the base of the websterite layer, where newly formed omphacite, quartz, and orthopyroxene replaced in part initial pyroxenes. Orthopyroxene and phlogopite (or an unidentified potassic phase) were formed in the reaction zone at the base of the harzburgite layer; among the initial minerals, only orthopyroxene relicts were preserved. Above the reaction zones produced by diffusion metasomatism, new phases developed locally, mainly at grain boundaries: newly formed orthopyroxene and magnesite were observed in harzburgite, and omphacite and quartz, in websterite. Alterations along grain boundaries extended much further than the reaction zones, which indicates that fluid infiltration dominated over diffusion in the experiments. The experiments demonstrated that the H2O–CO2 fluid with dissolved major components released from the glaucophane schist can produce mineral assemblages of different chemical compositions in mantle materials: Na-bearing in websterite and K-bearing in harzburgite. The complementary components, K2O and CO2 for the websterite layer and Na2O for the harzburgite layer, are fixed in the initial glaucophane schist layer. The distinguished separation of alkalis and CO2 at the crust–mantle boundary can affect the character of metasomatism in the mantle wedge, primary magma compositions, and the chemical evolution of the rocks of the subducting slab.








Similar content being viewed by others
Notes
The names of respective rocks, websterite and harzburgite, were used for the mixtures.
REFERENCES
Ague, J.J. and Nicolescu, S., Carbon dioxide released from subduction zones by fluid-mediated reactions, Nature Geosci., 2014, vol. 7, pp. 355–360.
Arai, S. and Ishimaru, S., Insights into petrological characteristics of the lithosphere of mantle wedge beneath arcs through peridotite xenoliths: a review, J. Petrol., 2008, vol. 49, no. 4, pp. 665–695.
Bali, E., Zajacz, Z., Kovacs, I., et al., A quartz-bearing orthopyroxene-rich websterite xenolith from the Pannonian Basin, western Hungary: evidence for release of quartz-saturated melt from a subducted slab, J. Petrol., 2008, vol. 49, pp. 421–439.
Bebout, G.E., Metamorphic chemical geodynamics of subduction zones, Earth Planet. Sci. Lett., 2007, vol. 260, pp. 373–393.
Berly, T.J., Hermann, J., Arculus, R.J., and Lapierre, H., Supra-subduction zone pyroxenites from San Jorge and Santa Isabel (Solomon Islands), J. Petrol., 2006, vol. 47, pp. 1531–1555.
Bose, K. and Ganguly, J., Quartz–coesite transition revisited: reversed experimental determination at 500–1200-degrees-C and retrieved thermochemical properties, Am. Mineral., 1995, vol. 80, pp. 231–238.
Bulatov, V.K., Brey, G.P., Girnis, A.V., et al., Carbonated sediment–peridotite interaction and melting at 7.5–12 GPa, Lithos, 2014, vol. 200–201, pp. 368–385.
Cloos, M., Flow melanges: numerical modeling and geologic constraints on their origin in the Franciscan subduction complex, California, Geol. Soc. Am. Bull., 1982, vol. 93, pp. 330–345.
Comodi, P., Nazzareni, S., Fumagalli, P., and Capitani, G.C., The peculiar crystal-chemistry of phlogopite from metasomatized peridotites: evidence from laboratory and nature, Period. Mineral., 2011, vol. 80, pp. 181–197.
Connolly, J.A.D., Computation of phase equilibria by linear programming: a tool for geodynamic modeling and its application to subduction zone decarbonation, Earth Planet. Sci. Lett., 2005, vol. 236, pp. 524–541.
Ertan, I.E. and Leeman, W.P., Metasomatism of Cascades subarc mantle: evidence from a rare phlogopite orthopyroxenite xenolith, Geology, 1996, vol. 24, pp. 451–454.
Frezzotti, M.L., Selverstone, J., Sharp, Z.D., and Compagnoni, R., Carbonate dissolution during subduction revealed by diamond-bearing rocks from the Alps, Nature Geosci., 2011, vol. 4, pp. 703–706.
Gervasoni, F., Klemme, S., Rohrbach, A., et al., Experimental constraints on mantle metasomatism caused by silicate and carbonate melts, Lithos, 2017, vol. 282–283, pp. 173–186.
Gerya, T., Future directions in subduction modeling, J. Geodynamics, 2011, vol. 52, pp. 344–378.
Gerya, T.V., Stoeckhert, B., and Perchuk, A.L., Exhumation of high-pressure metamorphic rocks in a subduction channel: a numerical simulation, Tectonics, 2002, vol. 21, no. 6, doi 10.1029/2002TC001406
Grant, T., Harlov, D.E., and Rhede, D., Experimental formation of pyroxenite veins by reactions between olivine and Si, Al, Ca, Na, and Cl-rich fluids at 800oC and 800 MPa: implications for fluid metasomatism in the mantle wedge, Am. Mineral., 2016, vol. 101, pp. 808–818.
Guillot, S., Hattori, K., Agard, P., et al., Exhumation processes in oceanic and continental subduction contexts: a review, Subduction Zone Geodynamics, Lallemand, S. and Funiciello, F., Eds., Heidelberg and Berlin: Springer, 2009.
Hermann, J., Spandler, C., Hack, A., and Korsakov, A.V., Aqueous fluids and hydrous melts in high-pressure and ultra-high pressure rocks: implications for element transfer in subduction zones, Lithos, 2006, vol. 92, pp. 399–417.
Holland, T. and Powell, R., Thermodynamics of order–disorder in minerals. 2. Symmetric formalism applied to solid solutions, Am. Mineral., 1996, vol. 81, pp. 1425–1437.
Holland, T.J.B. and Powell, R., An internally consistent thermodynamic data set for phases of petrological interest, J. Metamorph. Geol., 1998, vol. 16, pp. 309–343.
John, T., Klemd, R., Gao, J., and Garbe-Schonberg, C.D., Trace-element mobilization in slabs due to non steady-state fluid–rock interaction: constraints from an eclogite-facies transport vein in blueschist (Tianshan, China), Lithos, 2008, vol. 103, pp. 1–24.
Kepezhinskas, P.K., Defant, M.J., and Drummond, M.S., Na metasomatism in the island-arc mantle by slab–peridotite interaction: evidence from mantle xenoliths in the north Kamchatka arc, J. Petrol., 1995, vol. 36, pp. 1505–1527.
Khodorevskaya, L.I. and Zharikov, V.A., Experimental simulation of amphibolite and ultrabasic rock interaction in subduction zones, Petrology, 1997, vol. 5, pp. 2–8.
Korzhinskii, D.S., Fiziko-khimicheskie osnovy analiza paragenezisov mineralov (Physicochemical Basis of the Analysis of Mineral Parageneses), Moscow: AN SSSR, 1957.
Korzhinskii, D.S., Teoriya metasomaticheskoi zonal’nosti (Theory of Metasomatic Zoning), Moscow: Nauka, 1982.
Kretz, R., Symbols for rock-forming mineral, Am. Mineral., 1983, vol. 68, pp. 277–279.
Krogh Ravna, E. J., The garnet–clinopyroxene Fe2+–Mg geothermometer: an updated calibration, J. Metamorph. Geol., 2000, vol. 18, pp. 211–219.
Kushiro, I., The system forsterite–diopside–silica with and without water at high pressures, Am. J. Sci., 1969, vol. 267A, pp. 269–294.
Lambert, I.B. and Wyllie, P.J., Melting of gabbro (quartz eclogite) with excess water to 35 kilobars, with geological applications, J. Geol., 1972, vol. 80, pp. 693–708.
Malaspina, N., Hermann, J., Scambelluri, M., and Compagnoni, R., Polyphase inclusions in garnet-orthopyroxenites (debie shan, china) as monitors for metasomatism and fluid-related trace element transfer in subduction zone peridotite, Earth Planet. Sci. Lett., 2006, vol. 249, pp. 173–187.
Manning, C.E., The chemistry of subduction-zone fluids, Earth Planet. Sci. Lett., 2004, vol. 223, pp. 1–16.
O’Reilly, S.Y. and Griffin, W.L., Mantle metasomatism, Metasomatism and the Chemical Transformation of Rock, Harlov, D.E. and Austrheim, H., Eds., Lecture Notes Earth Syst. Sci., Berlin and Heidelberg: Springer, 2013.
Parman, S.W. and Grove, T.L., Harzburgite melting with and without H2O: experimental data and predictive modeling, J. Geophys. Res., 2004, vol. 109, B02201, doi 10.1029/2003JB002566
Patiño Douce, A.E. and Harris, N., Experimental constraints on Himalayan anatexis, J. Petrol., 1998, vol. 39, pp. 689–710.
Perchuk, A.L. and Korepanova, O.S., The problem of CO2 recycling in subduction zones, Mosk. Univ. Geol. Bull., 2011, vol. 66, no. 4, pp. 250–260.
Perchuk, A.L. and Yapaskurt, V.O., Experimental simulation of orthopyroxene enrichment and carbonation in the suprasubduction mantle under the influence of H2O, CO2, and SiO2, Geochem. Int., 2013, vol. 51, no. 4, pp. 257–268.
Perchuk, A.L., Shur, M.Yu., Yapaskurt, V.O., and Podgornova, S.T., Experimental modeling of mantle metasomatism coupled with eclogitization of crustal material in a subduction zone, Petrology, 2013, vol. 21, no. 6, pp. 579–598.
Perchuk, A.L., Yapaskurt, V.O., Griffin, W.G., et al., Three types of element fluxes from metabasite into peridotite in analogue experiments: insights into subduction-zone processes, Lithos, 2018, vol. 302–303, pp. 203–223.
Pirard, C. and Hermann, J., Focused fluid transfer through the mantle above subduction zones, Geology, 2015a, vol. 43, pp. 915–918.
Pirard, C. and Hermann, J., Experimentally determined stability of alkali amphibole in metasomatised dunite at sub-arc pressures, Contrib. Mineral. Petrol., 2015b, vol. 169, no. 1, pp. 1–26.
Powell, R. and Holland, T., Relating formulations of the thermodynamics of mineral solid solutions: activity modeling of pyroxenes, amphiboles, and micas, Am. Mineral., 1999, vol. 84, pp. 1–14.
Rapp, R.P., Shimizu, N., and Norman, M.D., Reaction between slab-derived melts and peridotite in the mantle wedge: experimental constraints at 3.8 GPa, Chem. Geol., 1999, vol. 160, pp. 335–356.
Ridley, J., Evidence of a temperature-dependent ‘blueschist’ to ‘eclogite’ transformation in high-pressure metamorphism of metabasic rocks, J. Petrol., 1984, vol. 25, pp. 852–870.
Safonov, O.G. and Butvina, V.G., Interaction of model peridotite with H2O–KCl fluid: experiment at 1.9 GPa and its implications for upper mantle metasomatism, Petrology, 2013, vol. 21, no. 6, pp. 599–615.
Scambelluri, M. and Philippot, P., Deep fluids in subduction zones, Lithos, 2001, vol. 55, pp. 213–227.
Schmidt, M.W. and Poli, S., Devolatilization during subduction, Treatise on Geochemistry, Holland H.D., and Turekian K.K., Eds., 2014, pp. 669–701.
Sekine, T. and Wyllie, P.J., The system granite–peridotite–H2O at 30 kbar, with applications to hybridization in subduction zone magmatism, Contrib. Mineral. Petrol., 1982, vol. 81, pp. 190–202.
Sobolev, N.V., Sobolev, V.N., Snyder, G.A., et al., Significance of eclogitic and related parageneses of natural diamonds, Int. Geol. Rev., 1999, vol. 41, pp. 129–140.
Spandler, C. and Pirard, C., Element recycling from subducting slabs to arc crust: a review, Lithos, 2013, vol. 170–171, pp. 208–223.
Syracuse, E.M., van Keken, P.E., and Abers, G.A., The global range of subduction zone thermal models, Phys. Earth Planet. Inter., 2010, vol. 183, no. 1, pp. 73–90.
van Keken, P.E., Hacker, B.R., Syracuse, E.M., and Abers, G.A., Subduction factory: 4. Depth-dependent flux of H2O from subducting slabs worldwide, J. Geophys. Res., 2011, vol. 116, B01401, http://dx.doi.org/. doi 10.1029/2010JB007922
White, R.W., Powell, R., Holland, T.J.B., et al., New mineral activity–composition relations for thermodynamic calculations in metapelitic systems, J. Metamorph. Geol., 2014, vol. 32, pp. 261–286.
Woodland, A.B., Bulatov, V.K., Brey, G.P., et al., Subduction factory in an ampoule: experiments on sediment–peridotite interaction under temperature gradient conditions, Geochim. Cosmochim. Acta, 2018, vol. 223, pp. 319–349.
Zheng, Y.F., Chen, R.X., Xu, Z., and Zhang, S.B., The transport of water in subduction zones, Sci. China Earth Sci., 2016, vol. 59, pp. 651–682.
ACKNOWLEDGMENTS
We are grateful to V.M. Polukeev (Institute of Experimental Mineralogy, Russian Academy of Sciences) for help in the experimental work, S.T. Podgornova for contribution to the preparation of the manuscript, E.N. Gramenitskii (Faculty of Geology, Moscow State University), O.G. Safonov (Institute of Experimental Mineralogy, Russian Academy of Sciences), E.M. Spiridonov, and B.B. Shkurskii (Faculty of Geology, Moscow State University) for the discussion of our results. The constructive review of A.V. Girnis (Institute of Geology of Ore Deposits, Petrography, Mineralogy, and Geochemistry, Russian Academy of Sciences) helped to improve the manuscript. Equipment purchased with funds from the Program of the Development of the Moscow State University was used in our investigations. This study was financially supported by the Russian Foundation for Basic Research, project no. 16-05-00495.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Girnis
Rights and permissions
About this article
Cite this article
Perchuk, A.L., Yapaskurt, V.O., Zinovieva, N.G. et al. Experimental Evidence for Opposite Fluxes of Sodium, Potassium, and CO2 during Glaucophane Schist Interaction with Harzburgite and Websterite in Subduction Zones. Petrology 26, 599–616 (2018). https://doi.org/10.1134/S086959111806005X
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S086959111806005X