Abstract
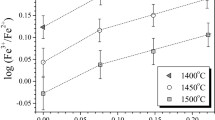
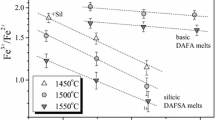
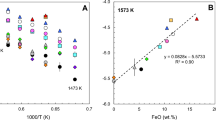
The solubility of cobalt and iron in silicate melts with variable SiO2 content was experimentally determined under controlled oxygen fugacity. It was shown that, independent of temperature and oxygen fugacity, the solubility of the two metals reaches a maximum (minimum of CoO and FeO activity coefficients) in melts of intermediate compositions. The analysis of available published data demonstrated that the γMeO values of at least four metals (Ni, Co, Fe, and Cr) dissolving in melts as divalent oxides show a minimum in melts with \(X_{SiO_2 } \) ≈ 57 ± 2 mol %. The position of the minimum is essentially independent of the element, melt temperature, and oxide concentration (from a few ppm to 13 wt%). The extremes of iron solubility (γFeO) in Fe-rich MgO-free melts may shift toward significantly lower \(X_{SiO_2 } \) values, although this inference requires additional experimental verification. Using a numerical example, some problems were discussed in the use of experimental data obtained in different laboratories for the development of a general model for the γMeO dependence on melt composition.
Similar content being viewed by others
References
A. Borisov, “Loop Technique: Dynamic of Metal/Melt Equilibration,” Mineral. Petrol. 71, 87–94 (2001).
A. A. Borisov, “Experimental Study of the Effect of SiO2 on Ni Solubility in Silicate Melts,” Petrologiya 14, 564–575 (2006) [Petrology 14, 530–539 (2006)].
A. Borisov, Y. Lahaye, and H. Palme, “The Effect of Sodium on the Solubilities of Metals in Silicate Melts,” Am. Mineral. 91, 762–771 (2006).
C. Doyle, “Prediction of the Activity of FeO in Multicomponent Magma from Known Values in [SiO2-KAlO2-CaAl2Si2O8]-FeO Liquids,” Geochim. Cosmochim. Acta 52, 1827–1834 (1988).
C. Doyle, “Effect of Substitution of TiO2 for SiO2 on a FeO in Magma,” Geochim. Cosmochim. Acta 53, 2631–2638 (1989).
C. Doyle and A. J. Naldrett, “Ideal Mixing of Divalent Cations in Mafic Magma and Its Effect on the Solution of Ferrous Oxide,” Geochim. Cosmochim. Acta 50, 435–443 (1986).
A. Holzheid, H. Palme, and S. Chakraborty, “The Activities of NiO, CoO and FeO in Silicate Melts,” Chem. Geol. 139, 21–38 (1997).
H. St. C. O’Neill and A. J. Berry, “Activity Coefficients at Low Dilution of CrO, NiO and CoO in Melts in the System CaO-MgO-Al2O3-SiO2 at 1400°C: Using the Thermodynamic Behavior of Transition Metal Oxides in Silicate Melts to Probe Their Structure,” Chem. Geol. 231, 77–89 (2006).
H. St. C. O’Neill and S. M. Eggins, “The Effect of Melt Composition on Trace Element Partitioning: An Experimental Investigation of the Activity Coefficients of FeO, NiO, CoO, MoO2 and MoO3 in Silicate Melts,” Chem. Geol. 186, 151–181 (2002).
E. B. Pretorius and A. Muan, “Activity of Nickel (II) Oxide in Silicate Melts,” J. Am. Ceram. Soc. 75, 1490–1496 (1992).
R. O. Sack, I. S. E. Carmichael, M. Rivers, and M. S. Ghiorso, “Ferric-Ferrous Equilibria in Natural Silicate Liquids at 1 Bar,” Contrib. Mineral. Petrol. 75, 369–376 (1980).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.A. Borisov, 2007, published in Petrologiya, 2007, Vol. 15, No. 6, pp. 563–570.
Rights and permissions
About this article
Cite this article
Borisov, A.A. Experimental study of the influence of SiO2 on the solubility of cobalt and iron in silicate melts. Petrology 15, 523–529 (2007). https://doi.org/10.1134/S086959110706001X
Received:
Issue Date:
DOI: https://doi.org/10.1134/S086959110706001X