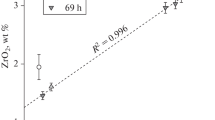
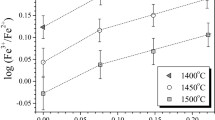
Abstract
The solubility of Ni in silicate melts with variable SiO2 content was studied at a total pressure of 1 atm within a wide range of temperature and oxygen fugacity. The maximum solubility of Ni (minimum activity coefficient of NiO) was observed in melts with ∼55–57 wt % SiO2, regardless of temperature and oxygen fugacity. Melts beyond this range showed significantly lower Ni solubility and, correspondingly, higher NiO activity coefficients. The analysis of our results and literature data led us to the conclusion that the NBO/T (number of nonbridging oxygen atoms per tetrahedrally coordinated atom) is inadequate to describe the effect of melt composition on Ni solubility.
Similar content being viewed by others
References
E. D. Andreeva, V. A. Baskina, O. A. Bogatikov, et al., Igneous Rocks: Classification, Nomenclature, and Petrography (Nauka, Moscow, 1983) [in Russian].
P. Beattie, C. Ford, and D. Russel, “Partition Coefficients for Olivine-Melt and Orthopyroxene-Melt Systems,” Contrib. Mineral. Petrol. 109, 212–224 (1991).
A. Borisov, “Loop Technique: Dynamics of Metal/Melt Equilibration,” Mineral. Petrol. 71, 87–94 (2001).
A. Borisov, Y. Lahaye, and H. Palme, “The Effect of TiO2 on Pd, Ni and Fe Solubilities in Silicate Melts,” Am. Mineral. 89, 564–571 (2004).
S. P. Clark, J. F. Schairer, Jr., and J. De Neufville, “Phase Relations in the System CaMgSi2O6—CaAl2SiO6-SiO2 at Low and High Pressure,” Carnegie Inst. Washington, Year Book 61, 59–68 (1962).
R. O. Colson, A. M. Floden, T. R. Haugen, et al., “Activities of NiO, FeO, and O2− in Silicate Melts,” Geochim. Cosmochim. Acta 69, 3061–3073 (2005).
P. S. Deines, R. H. Nafziger, G. C. Ulmer, and E. Woermann, “Temperature-Oxygen Fugacity Tables for Selected Gas Mixtures in the System C-H-O at One Atmosphere Total Pressure,” Bull. Earth Miner. Sci., Exp. St. 88 (1974).
D. B. Dingwell, H. St. C. O’Neill, W. Ertel, and B. Spettel, “The Solubility and Oxidation State of Ni in Silicate Melts at Low Oxygen Fugacities: Results Using a Mechanically Assisted Equilibration Technique,” Geochim. Cosmochim. Acta 58, 1967–1974 (1994).
W. Ertel, D. B. Dingwell, and H. St. C. O’Neill, “Solubility of Tungsten in a Haplobasaltic Melt as Function of Temperature and Oxygen Fugacity,” Geochim. Cosmochim. Acta 60, 1171–1180 (1996).
W. Ertel, D. B. Dingwell, and H. St. C. O’Neill, “Compositional Dependence of the Activity of Nickel in Silicate Melts,” Geochim. Cosmochim. Acta 61, 4707–4721 (1997).
A. Holzheid, H. Palme, and S. Chakraborty, “The Activities of NiO, CoO and FeO in Silicate Melts,” Chem. Geol. 139, 21–38 (1997).
J. E. Mungall, “Empirical Models Relating Viscosity and Tracer Diffusion in Magmatic Silicate Melts,” Geochim. Cosmochim. Acta 66, 125–143 (2002).
B. O. Mysen, Structure and Properties of Silicate Melts (Elsevier, Amsterdam, 1988).
G. S. Nikolaev, A. A. Borisov, and A. A. Ariskin, “Calculation of the Ferric-Ferrous Ratio in Magmatic Melts: Testing and Additional Calibration of Empirical Equations for Various Magatic Series,” Geokhimiya, No. 8, 713–722 (1996) [Geochem. Int. 34, 641–649 (1996)].
H. St. C. O’Neill and S. M. Eggins, “The Effect of Melt Composition on Trace Element Partitioning: An Experimental Investigation of the Activity Coefficients of FeO, NiO, CoO, MoO2 and MoO3 in Silicate Melts,” Chem. Geol. 186, 151–181 (2002).
G. Ottonello, R. Moretti, L. Marini, and M. V. Zuccolini, “Oxidation State of Iron in Silicate Glasses and Melts: A Thermochemical Model,” Chem. Geol. 174, 157–179 (2001).
E. B. Pretorius and A. Muan, “Activity of Nickel (II) Oxide in Silicate Melts,” J. Am. Ceram. Soc. 75, 1490–1496 (1992).
K. Righter, M. J. Drake, and G. Yaxley, “Prediction of Siderophile Element Metal-Silicate Partitioning Coefficients to 20 GPa and 2800°C: the Effect of Pressure, Temperature, Oxygen Fugacity, and Silicate and Metallic Melt Compositions,” Phys. Earth Planet. Inter. 100, 115–134 (1997).
R. O. Sack, I. S. E. Carmichael, M. Rivers, and N. S. Ghiorso, “Ferric-Ferrous Equilibria in Natural Silicate Liquids at 1 bar,” Contrib. Mineral. Petrol. 75, 369–376 (1980).
M. Temkin, “Mixtures of Molten Salts as Ionic Solutions,” Zh. Fiz. Khim. 20(1), 105–110 (1946).
G. W. Toop and C. S. Samis, “Activities of Ions in Silicate Melts,” Trans. Metall. Soc. AIME 224, 878–887 (1962).
Author information
Authors and Affiliations
Additional information
Original Russian Text © A.A. Borisov, 2006, published in Petrologiya, 2006, Vol. 14, No. 6, pp. 564–575.
Rights and permissions
About this article
Cite this article
Borisov, A.A. Experimental study of the effect of SiO2 on Ni solubility in silicate melts. Petrology 14, 530–539 (2006). https://doi.org/10.1134/S0869591106060026
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0869591106060026