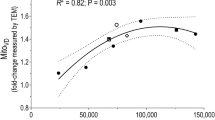
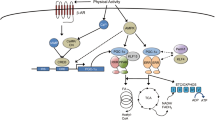
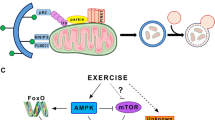
Abstract
Physical inactivity and disuse lead to a decrease in the functionality of skeletal muscles (oxidative capacity, insulin sensitivity, and performance), which is associated with a change in mitochondrial density. In contrast, aerobic exercise training is effective for maintaining/increasing skeletal muscle mitochondrial density and functionality. The review considers the effect of increasing and decreasing physical activity on the mitochondrial density of human skeletal muscles, as well as the main mechanisms responsible for these changes. It is discussed that the content of mitochondrial proteins can be regulated by changing the content of their mRNAs, changes in the rate of synthesis specific for mitochondrial proteins, as well as changes in the rate of degradation, transport, import, and stability of mitochondrial proteins. It has been shown that the mechanisms of regulation of the mitochondrial proteins content under various interventions are significantly different. At the same time, their contribution to the change in the content of mitochondrial proteins is characterized clearly insufficiently, which emphasizes the relevance of further research in this area.
Similar content being viewed by others
REFERENCES
Lanza, I.R., Short, D.K., Short, K.R., et al., Endurance exercise as a countermeasure for aging, Diabetes, 2008, vol. 57, no. 11, p. 2933.
Pedersen, B.K. and Febbraio, M.A., Muscles, exercise and obesity: skeletal muscle as a secretory organ, Nat. Rev. Endocrinol., 2012, vol. 8, no. 8, p. 457.
Demontis, F., Piccirillo, R., Goldberg, A.L., and Perrimon, N., The influence of skeletal muscle on systemic aging and lifespan, Aging Cell, 2013, vol. 12, no. 6, p. 943.
Agudelo, L.Z., Femenía, T., Orhan, F., et al., Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression, Cell, 2014, vol. 159, no. 1, p. 33.
Waller, L., Krüger, K., Conrad, K., et al., Effects of different types of exercise training on pulmonary arterial hypertension: a systematic review, J. Clin. Med., 2020, vol. 9, no. 6, p. 1689.
Schild, M., Ruhs, A., Beiter, T., et al., Basal and exercise induced label-free quantitative protein profiling of m. vastus lateralis in trained and untrained individuals, J. Proteomics, 2015, vol. 122, p. 119.
Makhnovskii, P.A., Zgoda, V.G., Bokov, R.O., et al., Regulation of proteins in human skeletal muscle: the role of transcription, Sci. Rep., 2020, vol. 10, no. 1, p. 3514.
Saltin, B., Henriksson, J., Nygaard, E., et al., Fiber types and metabolic potentials of skeletal muscles in sedentary man and endurance runners, Ann. N.Y. Acad. Sci., 1977, vol. 301, p. 3.
San-Millán, I. and Brooks, G.A., Assessment of metabolic flexibility by means of measuring blood lactate, fat, and carbohydrate oxidation responses to exercise in professional endurance athletes and less-fit individuals, Sports Med., 2018, vol. 48, no. 2, p. 467.
Montero, D., Cathomen, A., Jacobs, R.A., et al., Haematological rather than skeletal muscle adaptations contribute to the increase in peak oxygen uptake induced by moderate endurance training, J. Physiol., 2015, vol. 593, no. 20, p. 4677.
Hawley, J.A., Hargreaves, M., Joyner, M.J., and Zierath, J.R., Integrative biology of exercise, Cell, 2014, vol. 159, no. 4, p. 738.
Tomilovskaya, E., Shigueva, T., Sayenko, D., et al., Dry immersion as a ground-based model of microgravity physiological effects, Front. Physiol., 2019, vol. 10, p. 284.
Sharlo, K., Tyganov, S.A., Tomilovskaya, E., et al., Effects of various muscle disuse states and countermeasures on muscle molecular signaling, Int. J. Mol. Sci., 2022, vol. 23, no. 1, p. 468.
Hackney, K.J. and Ploutz-Snyder, L.L., Unilateral lower limb suspension: integrative physiological knowledge from the past 20 years (1991–2011), Eur. J. Appl. Physiol., 2012, vol. 112, no. 1, p. 9.
Hyatt, H., Deminice, R., Yoshihara, T., and Powers, S.K., Mitochondrial dysfunction induces muscle atrophy during prolonged inactivity: a review of the causes and effects, Arch. Biochem. Biophys., 2019, vol. 662, p. 49.
Gram, M., Dahl, R., and Dela, F., Physical inactivity and muscle oxidative capacity in humans, Eur. J. Sport Sci., 2013, vol. 14, no. 4, p. 376.
Brocca, L., Cannavino, J., Coletto, L., et al., The time course of the adaptations of human muscle proteome to bed rest and the underlying mechanisms, J. Physiol., 2012, vol. 590, no. 20, p. 5211.
Hather, B.M., Adams, G.R., Tesch, P.A., and Dudley, G.A., Skeletal muscle responses to lower limb suspension in humans, J. Appl. Physiol., 1992, vol. 72, no. 4, p. 1493.
Berg, H.E., Dudley, G.A., Hather, B., and Tesch, P.A., Work capacity and metabolic and morphologic characteristics of the human quadriceps muscle in response to unloading, Clin. Physiol., 1993, vol. 13, no. 4, p. 337.
Salanova, M., Schiffl, G., Püttmann, B., et al., Molecular biomarkers monitoring human skeletal muscle fibers and microvasculature following long-term bed rest with and without countermeasures, J. Anat., 2008, vol. 212, no. 3, p. 306.
Vigelsø, A., Gram, M., Wiuff, C., et al., Six weeks’ aerobic retraining after two weeks’ immobilization restores leg lean mass and aerobic capacity but does not fully rehabilitate leg strength in young and older men, J. Rehabil. Med., 2015, vol. 47, no. 6, p. 552.
Rudnick, J., Püttmann, B., Tesch, P.A., et al., Differential expression of nitric oxide synthases (NOS 1–3) in human skeletal muscle following exercise countermeasure during 12 weeks of bed rest, FASEB J., 2004, vol. 18, no. 11, p. 1228.
Arentson-Lantz, E.J., English, K.L., Paddon-Jones, D., and Fry, C.S., Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle-aged adults, J. Appl. Physiol., 2016, vol. 120, no. 8, p. 965.
Narici, M.V. and de Boer, M.D., Disuse of the musculo-skeletal system in space and on earth, Eur. J. Appl. Physiol., 2011, vol. 111, no. 3, p. 403.
Bamman, M.M., Clarke, M.S.F., Feeback, D.L., et al., Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution, J. Appl. Physiol., 1998, vol. 84, no. 1, p. 157.
Hortobágyi, T., Dempsey, L., Fraser, D., et al., Changes in muscle strength, muscle fibre size and myofibrillar gene expression after immobilization and retraining in humans, J. Physiol., 2000, vol. 524, p. 293.
Yasuda, N., Glover, E.I., Phillips, S.M., et al., Sex-based differences in skeletal muscle function and morphology with short-term limb immobilization, J. Appl. Physiol., 2005, vol. 99, no. 3, p. 1085.
Hvid, L., Aagaard, P., Justesen, L., et al., Effects of aging on muscle mechanical function and muscle fiber morphology during short-term immobilization and subsequent retraining, J. Appl. Physiol., 2010, vol. 109, no. 6, p. 1628.
Hood, D.A., Memme, J.M., Oliveira, A.N., and Triolo, M., Maintenance of skeletal muscle mitochondria in health, exercise, and aging, Annu. Rev. Physiol., 2019, vol. 81, p. 19.
Popov, D.V., Adaptation of skeletal muscles to contractile activity of varying duration and intensity: the role of PGC-1α, Biochemistry (Moscow), 2018, vol. 83, no. 6, p. 613.
Perry, C.G.R. and Hawley, J.A., Molecular basis of exercise-induced skeletal muscle mitochondrial biogenesis: historical advances, current knowledge, and future challenges, Cold Spring Harb. Perspect. Med., 2018, vol. 8, no. 9, p. 1.
Memme, J.M., Erlich, A.T., Phukan, G., and Hood, D.A., Exercise and mitochondrial health, J. Physiol., 2021, vol. 599, no. 3, p. 803.
Olesen, J., Kiilerich, K., and Pilegaard, H., PGC-1α-mediated adaptations in skeletal muscle, Pflugers Arch., 2010, vol. 460, no. 1, p. 153.
Narkar, V.A., Fan, W., Downes, M., et al., Exercise and PGC-1α-independent synchronization of type i muscle metabolism and vasculature by ERRγ, Cell Metab., 2011, vol. 13, no. 3, p. 283.
Scarpulla, R.C., Transcriptional paradigms in mammalian mitochondrial biogenesis and function, Physiol. Rev., 2008, vol. 88, no. 2, p. 611.
Pearen, M.A. and Muscat, G.E.O., The nuclear receptor Nor-1 is a pleiotropic regulator of exercise-induced adaptations, Exercise Sports Sci. Rev., 2018, vol. 46, no. 2, p. 97.
Wu, Z., Huang, X., Feng, Y., et al., Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1α transcription and mitochondrial biogenesis in muscle cells, Proc. Natl. Acad. Sci. U.S.A., 2006, vol. 103, no. 39, p. 14379.
Pérez-Schindler, J., Summermatter, S., Salatino, S., et al., The corepressor NCoR1 antagonizes PGC-1α and estrogen-related receptor α in the regulation of skeletal muscle function and oxidative metabolism, Mol. Cell. Biol., 2012, vol. 32, no. 24, p. 4913.
Williams, R.S., Salmons, S., Newsholme, E.A., et al., Regulation of nuclear and mitochondrial gene expression by contractile activity in skeletal muscle, J. Biol. Chem., 1986, vol. 261, no. 1, p. 376.
Williams, R.S., Garcia-Moll, M., Mellor, J., et al., Adaptation of skeletal muscle to increased contractile activity. Expression nuclear genes encoding mitochondrial proteins, J. Biol. Chem., 1987, vol. 262, no. 6, p. 2764.
Morrison, P.R., Biggs, R.B., and Booth, F.W., Daily running for 2 wk and mRNAs for cytochrome c and α-actin in rat skeletal muscle, Am. J. Physiol., 1989, vol. 257, no. 5, p. C936.
Freyssenet, D., Connor, M.K., Takahashi, M., and Hood, D.A., Cytochrome c transcriptional activation and mRNA stability during contractile activity in skeletal muscle, Am. J. Physiol., 1999, vol. 277, no. 1, p. E26.
Hood, D.A., Zak, R., and Pette, D., Chronic stimulation of rat skeletal muscle induces coordinate increases in mitochondrial and nuclear mRNAs of cytochrome-c-oxidase subunits, Eur. J. Biochem., 1989, vol. 179, no. 2, p. 275.
Freyssenet D, Connor MK, Takahashi M, et al. Cytochrome c transcriptional activation and mRNA stability during contractile activity in skeletal muscle. Am. J. Physiol. 1999. V. 277. № 6. P E26.
Nishida, Y., Tanaka, H., Tobina, T., et al., Regulation of muscle genes by moderate exercise, Int. J. Sports Med., 2010, vol. 31, no. 9, p. 656.
Robinson, M.M., Dasari, S., Konopka, A.R., et al., Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans, Cell Metab., 2017, vol. 25, no. 3, p. 581.
Granata, C., Caruana, N.J., Botella, J., et al., Training-induced bioenergetic improvement in human skeletal muscle is associated with non-stoichiometric changes in the mitochondrial proteome without reorganization of respiratory chain content, Nat. Commun., 2021, vol. 12, no. 1, p. 7056.
Radom-Aizik, S., Hayek, S., Shahar, I., et al., Effects of aerobic training on gene expression in skeletal muscle of elderly men, Med. Sci. Sports Exercise, 2005, vol. 37, no. 10, p. 1680.
Chapman, M.A., Arif, M., Emanuelsson, E.B., et al., Skeletal muscle transcriptomic comparison between long-term trained and untrained men and women, Cell Rep., 2020, vol. 31, no. 12, p. 107808.
Pillon, N.J., Gabriel, B.M., Dollet, L., et al., Transcriptomic profiling of skeletal muscle adaptations to exercise and inactivity, Nat. Commun., 2020, vol. 11, no. 1, p. 470.
Keller, P., Vollaard, N.B.J., Gustafsson, T., et al., A transcriptional map of the impact of endurance exercise training on skeletal muscle phenotype, J. Appl. Physiol., 2011, vol. 110, no. 1, p. 46.
Turan, N., Kalko, S., Stincone, A., et al., A systems biology approach identifies molecular networks defining skeletal muscle abnormalities in chronic obstructive pulmonary disease, PLoS Comput. Biol., 2011, vol. 7, no. 9, p. e1002129.
Lindholm, M.E., Marabita, F., Gomez-Cabrero, D., et al., An integrative analysis reveals coordinated reprogramming of the epigenome and the transcriptome in human skeletal muscle after training, Epigenetics, 2014, vol. 9, no. 12, p. 1557.
Lindholm, M.E., Giacomello, S., Werne Solnestam, B., et al., The impact of endurance training on human skeletal muscle memory, global isoform expression and novel transcripts, PLoS Genet., 2016, vol. 12, no. 9, p. e1006294.
Clarke, K., Ricciardi, S., Pearson, T., et al., The role of Eif6 in skeletal muscle homeostasis revealed by endurance training co-expression networks, Cell Rep., 2017, vol. 21, no. 6, p. 1507.
Popov, D.V., Makhnovskii, P.A., Shagimardanova, E.I., et al., Contractile activity-specific transcriptome response to acute endurance exercise and training in human skeletal muscle, Am. J. Physiol.: Endocrinol. Metab., 2019, vol. 316, no. 4, p. E605.
Knuiman, P., Hangelbroek, R., Boekschoten, M., et al., Impact of protein supplementation during endurance training on changes in skeletal muscle transcriptome, BMC Genomics, 2020, vol. 21, no. 1, p. 397.
Makhnovskii, P.A., Bokov, R.O., Kolpakov, F.A., and Popov, D.V., Transcriptomic signatures and upstream regulation in human skeletal muscle adapted to disuse and aerobic exercise, Int. J. Mol. Sci., 2021, vol. 22, no. 3, p. 1208.
Liu, X., Zhang, Y., Ni, M., et al., Regulation of mitochondrial biogenesis in erythropoiesis by mTORC1-mediated protein translation, Nat. Cell Biol., 2017, vol. 19, no. 6, p. 626.
Cheng, Z., Teo, G., Krueger, S., et al., Differential dynamics of the mammalian mRNA and protein expression response to misfolding stress, Mol. Syst. Biol., 2016, vol. 12, no. 1, p. 855.
Catoire, M., Mensink, M., Boekschoten, M.V., et al., Pronounced effects of acute endurance exercise on gene expression in resting and exercising human skeletal muscle, PLoS One, 2012, vol. 7, no. 11, p. e51066.
McLean, C.S., Mielke, C., Cordova, J.M., et al., Gene and microRNA expression responses to exercise; relationship with insulin sensitivity, PLoS One, 2015, vol. 10, no. 5, p. e0127089.
Dickinson, J.M., D’Lugos, A.C., Naymik, M.A., et al., Transcriptome response of human skeletal muscle to divergent exercise stimuli, J. Appl. Physiol., 2018, vol. 124, no. 6, p. 1529.
Vissing, K. and Schjerling, P., Simplified data access on human skeletal muscle transcriptome responses to differentiated exercise, Sci. Data, 2014, vol. 1, p. 140041.
Rowlands, D.S., Thomson, J.S., Timmons, B.W., et al., Transcriptome and translational signaling following endurance exercise in trained skeletal muscle: impact of dietary protein, Physiol. Genomics, 2011, vol. 43, no. 17, p. 1004.
Neubauer, O., Sabapathy, S., Ashton, K.J., et al., Time course-dependent changes in the transcriptome of human skeletal muscle during recovery from endurance exercise: from inflammation to adaptive remodeling, J. Appl. Physiol., 2014, vol. 116, no. 3, p. 274.
Popov, D.V., Makhnovskii, P.A., Kurochkina, N.S., et al., Intensity-dependent gene expression after aerobic exercise in endurance-trained skeletal muscle, Biol. Sports, 2018, vol. 35, no. 3, p. 277.
Rudler, D.L., Hughes, L.A., Perks, K.L., et al., Fidelity of translation initiation is required for coordinated respiratory complex assembly, Sci. Adv., 2019, vol. 5, no. 12, p. eaay2118.
Rudler, D.L., Hughes, L.A., Viola, H.M., et al., Fidelity and coordination of mitochondrial protein synthesis in health and disease, J. Physiol., 2021, vol. 599, no. 14, p. 3449.
Khalimonchuk, O., Bird, A., and Winge, D.R., Evidence for a pro-oxidant intermediate in the assembly of cytochrome oxidase, J. Biol. Chem., 2007, vol. 282, no. 24, p. 17442.
Hu, D., Liu, Z., Qi, X., and Perez, M.J., Mitochondrial quality control strategies: potential therapeutic targets for neurodegenerative diseases? Front. Neurosci., 2021, vol. 15, p. 746873.
Chopard, A., Lecunff, M., Danger, R., et al., Large-scale mRNA analysis of female skeletal muscles during 60 days of bed rest with and without exercise or dietary protein supplementation as countermeasures, Physiol. Genomics, 2009, vol. 38, no. 3, p. 291.
Abadi, A., Glover, E.I., Isfort, R.J., et al., Limb immobilization induces a coordinate down-regulation of mitochondrial and other metabolic pathways in men and women, PLoS One, 2009, vol. 4, no. 8, p. e6518.
Reich, K.A., Chen, Y.W., Thompson, P.D., et al., Forty-eight hours of unloading and 24 h of reloading lead to changes in global gene expression patterns related to ubiquitination and oxidative stress in humans, J. Appl. Physiol., 2010, vol. 109, no. 5, p. 1404.
Alibegovic, A.C., Sonne, M.P., Højbjerre, L., et al., Insulin resistance induced by physical inactivity is associated with multiple transcriptional changes in skeletal muscle in young men, Am. J. Physiol.: Endocrinol. Metab., 2010, vol. 299, no. 5, p. 752.
Lammers, G., Poelkens, F., Duijnhoven, N.T.L., et al., Expression of genes involved in fatty acid transport and insulin signaling is altered by physical inactivity and exercise training in human skeletal muscle, Am. J. Physiol.: Endocrinol. Metab., 2012, vol. 303, no. 10, p. e1245.
Rullman, E., Fernandez-Gonzalo, R., Mekjavić, I.B., et al., MEF2 as upstream regulator of the transcriptome signature in human skeletal muscle during unloading, Am. J. Physiol.: Regul. Integr. Comp. Physiol., 2018, vol. 315, no. 4, p. R799.
Trevino, M.B., Zhang, X., Standley, R.A., et al., Loss of mitochondrial energetics is associated with poor recovery of muscle function but not mass following disuse atrophy, Am. J. Physiol.: Endocrinol. Metab., 2019, vol. 317, no. 5, p. E899.
Mahmassani, Z.S., Reidy, P.T., McKenzie, A.I., et al., Age-dependent skeletal muscle transcriptome response to bed rest-induced atrophy, J. Appl. Physiol., 2019, vol. 126, no. 4, p. 894.
Standley, R.A., Distefano, G., Trevino, M.B., et al., Skeletal muscle energetics and mitochondrial function are impaired following 10 days of bed rest in older adults, J. Gerontol. A, 2020, vol. 75, no. 9, p. 1744.
Fernandez-Gonzalo, R., Tesch, P.A., Lundberg, T.R., et al., Three months of bed rest induce a residual transcriptomic signature resilient to resistance exercise countermeasures, FASEB J., 2020, vol. 34, no. 6, p. 7958.
Migliavacca, E., Tay, S.K.H., Patel, H.P., et al., Mitochondrial oxidative capacity and NAD+ biosynthesis are reduced in human sarcopenia across ethnicities, Nat. Commun., 2019, vol. 10, no. 1, p. 5808.
Wilkinson, S.B., Phillips, S.M., Atherton, P.J., et al., Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle, J. Physiol., 2008, vol. 586, no. 15, p. 3701.
Donges, C.E., Burd, N.A., Duffield, R., et al., Concurrent resistance and aerobic exercise stimulates both myofibrillar and mitochondrial protein synthesis in sedentary middle-aged men, J. Appl. Physiol., 2012, vol. 112, no. 12, p. 1992.
Leppek, K., Das, R., and Barna, M., Functional 5′ UTR mRNA structures in eukaryotic translation regulation and how to find them, Nat. Rev. Mol. Cell Biol., 2018, vol. 19, no. 3, p. 158.
Sinvani, H., Haimov, O., Svitkin, Y., et al., Translational tolerance of mitochondrial genes to metabolic energy stress involves TISU and eIF1-eIF4GI cooperation in start codon selection, Cell Metab., 2015, vol. 21, no. 3, p. 479.
Morita, M., Gravel, S.P., Chénard, V., et al., mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation, Cell Metab., 2013, vol. 18, no. 5, p. 698.
Haimov, O., Sinvani, H., and Dikstein, R., Cap-dependent, scanning-free translation initiation mechanisms, Biochim. Biophys. Acta, Gene Regul. Mech., 2015, vol. 1849, no. 11, p. 1313.
Elfakess, R. and Dikstein, R., A translation initiation element specific to mRNAs with very short 5′UTR that also regulates transcription, PLoS One, 2008, vol. 3, no. 8, p. e3094.
Rudrappa, S.S., Wilkinson, D.J., Greenhaff, P.L., et al., Human skeletal muscle disuse atrophy: effects on muscle protein synthesis, breakdown, and insulin resistance—A qualitative review, Front. Physiol., 2016, vol. 7, p. 361.
Crossland, H., Skirrow, S., Puthucheary, Z.A., et al., The impact of immobilization and inflammation on the regulation of muscle mass and insulin resistance: different routes to similar end-points, J. Physiol., 2019, vol. 597, no. 5, p. 1259.
Bragoszewski, P., Turek, M., and Chacinska, A., Control of mitochondrial biogenesis and function by the ubiquitin-proteasome system, Open Biol., 2017, vol. 7, no. 4, art. ID 170007.
Lavie, J., De Belvalet, H., Sonon, S., et al., Ubiquitin-dependent degradation of mitochondrial proteins regulates energy metabolism, Cell Rep., 2018, vol. 23, no. 10, p. 2852.
Johnson, M.L., Robinson, M.M., and Nair, S.K., Skeletal muscle aging and the mitochondrion, Trends Endocrinol. Metab., 2013, vol. 24, no. 5, p. 247.
Louis, E., Raue, U., Yang, Y., et al., Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle, J. Appl. Physiol., 2007, vol. 103, no. 5, p. 1744.
Harber, M.P., Crane, J.D., Dickinson, J.M., et al., Protein synthesis and the expression of growth-related genes are altered by running in human vastus lateralis and soleus muscles, Am. J. Physiol.: Regul. Integr. Comp. Physiol., 2009, vol. 296, no. 3, p. 708.
Pasiakos, S.M., McClung, H.L., McClung, J.P., et al., Molecular responses to moderate endurance exercise in skeletal muscle, Int. J. Sport Nutr. Exercise Metab., 2010, vol. 20, no. 4, p. 282.
Stefanetti, R.J., Lamon, S., Wallace, M., et al., Regulation of ubiquitin proteasome pathway molecular markers in response to endurance and resistance exercise and training, Pflugers Arch., 2015, vol. 467, no. 7, p. 1523.
Møller, A.B., Vendelbo, M.H., Christensen, B., et al., Physical exercise increases autophagic signaling through ULK1 in human skeletal muscle, J. Appl. Physiol., 2015, vol. 118, no. 8, p. 971.
Cunha, T.F., Moreira, J.B.N., Paixão, N.A., et al., Aerobic exercise training upregulates skeletal muscle calpain and ubiquitin-proteasome systems in healthy mice, J. Appl. Physiol., 2012, vol. 112, no. 11, p. 1839.
Kim, Y.A., Kim, Y.S., Oh, S.L., et al., Autophagic response to exercise training in skeletal muscle with age, J. Physiol. Biochem., 2013, vol. 69, no. 4, p. 697.
Kim, Y.A., Kim, Y.S., and Song, W., Autophagic response to a single bout of moderate exercise in murine skeletal muscle, J. Physiol. Biochem., 2012, vol. 68, no. 2, p. 229.
Popov, D.V., Lysenko, E.A., Miller, T.F., et al., The effect of single aerobic exercise on the regulation of mitochondrial biogenesis in skeletal muscles of trained men: a time-course study, Hum. Physiol., 2015, vol. 41, no. 3, p. 296.
Young, J.C., Hoogenraad, N.J., and Hartl, F.U., Molecular chaperones Hsp90 and Hsp70 deliver pre-proteins to the mitochondrial import receptor Tom70, Cell, 2003, vol. 112, no. 1, p. 41.
Evgen’ev, M.B., Garbuz, D.G., and Zatsepina, O.G., Heat shock proteins: functions and role in adaptation to hyperthermia, Russ. J. Dev. Biol., 2005, vol. 36, no. 4, p. 218.
Williamson, C.L., Dabkowski, E.R., Dillmann, W.H., and Hollander, J.M., Mitochondria protection from hypoxia/reoxygenation injury with mitochondria heat shock protein 70 overexpression, Am. J. Physiol.: Hear. Circ. Physiol., 2008, vol. 294, no. 1, p. 249.
Shepherd, D.L., Hathaway, Q.A., Nichols, C.E., et al., Mitochondrial proteome disruption in the diabetic heart through targeted epigenetic regulation at the mitochondrial heat shock protein 70 (mtHsp70) nuclear locus, J. Mol. Cell. Cardiol., 2018, vol. 119, p. 104.
Ma, X., Xu, L., Alberobello, A.T., et al., Celastrol protects against obesity and metabolic dysfunction through activation of a HSF1-PGC1α transcriptional axis, Cell Metab., 2015, vol. 22, no. 4, p. 695.
Takahashi, M., Chesley, A., Freyssenet, D., and Hood, D.A., Contractile activity-induced adaptations in the mitochondrial protein import system, Am. J. Physiol., 1998, vol. 274, no. 5, p. 1380.
Memme, J.M., Slavin, M., Moradi, N., and Hood, D.A., Mitochondrial bioenergetics and turnover during chronic muscle disuse, Int. J. Mol. Sci., 2021, vol. 22, no. 10, p. 5179.
Jones, S.W., Hill, R.J., Krasney, P.A., et al., Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass, FASEB J., 2004, vol. 18, no. 9, p. 1025.
Gustafsson, T., Osterlund, T., Flanagan, J.N., et al., Effects of 3 days unloading on molecular regulators of muscle size in humans, J. Appl. Physiol., 2010, vol. 109, no. 3, p. 721.
Møller, A.B., Vendelbo, M.H., Schjerling, P., et al., Immobilization decreases foxo3a phosphorylation and increases autophagy-related gene and protein expression in human skeletal muscle, Front. Physiol., 2019, vol. 10, p. 736.
Leermakers, P.A., Kneppers, A.E.M., Schols, A.M.W.J., et al., Skeletal muscle unloading results in increased mitophagy and decreased mitochondrial biogenesis regulation, Muscle Nerve, 2019, vol. 60, no. 6, p. 769.
Singh, K. and Hood, D.A., Effect of denervation-induced muscle disuse on mitochondrial protein import, Am. J. Physiol.: Cell Physiol., 2011, vol. 300, no. 1, p. C138.
Camera, D.M., Burniston, J.G., Pogson, M.A., et al., Dynamic proteome profiling of individual proteins in human skeletal muscle after a high-fat diet and resistance exercise, FASEB J., 2017, vol. 31, no. 12, p. 5478.
Funding
This study was supported by the Russian Science Foundation, project no. 21-15-00405.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This work does not contain any studies involving animals or human subjects performed by any of the authors.
CONFLICT OF INTERESTS
The authors declare that they do not have a conflict of interests.
Additional information
Translated by E. Babchenko
Rights and permissions
About this article
Cite this article
Bokov, R.O., Popov, D.V. Regulation of Mitochondrial Biogenesis in Human Skeletal Muscles Induced by Aerobic Exercise and Disuse. Hum Physiol 48, 261–270 (2022). https://doi.org/10.1134/S0362119722030033
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119722030033