Abstract
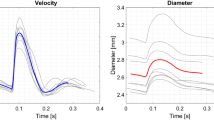
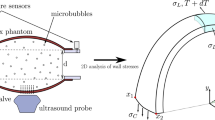
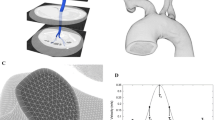
We performed an experimental study of the elastic properties of the aorta by several independent methods, including the elastometry of the porcine aorta in different stretching modes, aortography in an acute porcine experiment with various levels of blood pressure, and contrast multislice computed tomography (MSCT) of the aorta in a healthy volunteer. In previous studies, it was shown that the swirling nature of the blood flow in the heart chambers and the aorta may be described in terms of the hydrodynamic model of tornado-like self-organizing axisymmetric viscous fluid flows. The purpose of the study was to test the correspondence between the pattern of changes in the viscoelastic characteristics of the aorta walls and the conditions of the self-organization of the tornado-like flow in its lumen. Three independent methods, namely, postmortal elastometry, intravital panaortography, and MSCT of a healthy volunteer, have been used to show that the aorta elasticity monotonically decreases in the distal direction, this relationship being distorted with increasing radial load beyond the physiologically normal range. This distribution of elasticity along the aorta ensures that the geometric configuration of the aorta flow pass corresponds to the directions of the current lines of the tornado-like flow obtained from the precise solutions throughout the cardiac cycle. Thus, it has been established that the changes in the elastic properties along the aorta create the necessary hydrodynamic conditions for maintaining the tornado-like flow throughout the cardiac cycle in the physiological range of pressure.






Similar content being viewed by others
REFERENCES
Bockeria, L.A., Kiknadze, G.I., Gachechiladze, I.A., et al., Analysis of structure of intraventricular blood flow based on studies of architectonics of trabecular layer in left ventricle, Cardiometry J., 2013, vol. 3, p. 5.
Talygin, E.A., Zazybo, N.A., Zhorzholiani, S.T., et al., Quantitative evaluation of intracardiac blood flow by left ventricle dynamic anatomy based on exact solutions of non-stationary Navier-Stocks equations for self-organized tornado-like flows of viscous incompressible fluid, Usp. Fiziol. Nauk, 2016, vol. 47, no. 1, p. 48.
Bockeria, L.A., Kiknadze, G.I., Agafonov, A.V., et al., Application of tornado-flow fundamental hydrodynamic theory to the study of blood flow in the heart and main vessels: design of new implantable and accessory devices for cardiovascular surgery, ASME 2012 Int. Mechanical Engineering Congr. and Exposition (IMECE2012), New York: Am. Soc. Mech. Eng., 2012, vol. 2, p. 93.
Zhorzholiani, Sh.T., Mironov, A.A., Talygin, E.A., et al., Analysis of dynamic geometric configuration of the aortic channel from the perspective of tornado-like flow organization of blood flow, Bull. Exp. Biol. Med., 2018, vol. 164, no. 4, p. 514.
Kiknadze, G.I. and Krasnov, Yu.K., Evolution of a spout-like flow of a viscous fluid, Sov. Phys. Dokl., 1986, vol. 31, no. 10, p. 799.
Khamdaengyodtai, P., Terdtoon, P., and Sakulchangsatjatai, P., Three-dimension stress and strain distributions across five-layer human aortic wall, Int. J. Biosci., Biochem. Bioinf., 2013, vol. 3, no. 2, p. 177.
Menut, M., Bou-Said, B., Berre, H.W., et al., Characterization of the mechanical properties of the human aortic arch using an expansion method, J. Vasc. Med. Surg., 2015, vol. 3, p. 188.
Kassab, G.S. Biomechanics of the cardiovascular system: The aorta as an illustratory example, J. R. Soc. Interface, 2007, vol. 3, no. 11, p. 719.
Kim, J., Hong, J.W., and Baek, S., Longitudinal differences in the mechanical properties of the thoracic aorta depend on circumferential regions, J. Biomed. Mater. Res., Part A, 2013, vol. 101, no. 5, p. 1529.
Cavalcante, J.L., Lima, J.A., Redheuil, A., and Al-Mallah, M.H., Aortic stiffness: current understanding and future directions, J. Am. Coll. Cardiol., 2011, vol. 57, no. 14, p. 1511.
Bond, A.R., Iftikhar, S., Bharath, A.A., and Wein-berg, P.D., Morphological evidence for a change in the pattern of aortic wall shear stress with age, Arterioscler., Thromb., Vasc. Biol., 2011, vol. 31, no. 3, p. 543.
Wentland, A.L., Grist, T.M., and Wieben, O., Review of MRI-based measurements of pulse wave velocity: a biomarker of arterial stiffness, Cardiovasc. Diagn. Ther., 2014, vol. 4, no. 2, p. 193.
Pierce, D.M., Maier, F., Weisbecker, H., et al., Human thoracic and abdominal aortic aneurysmal tissues: damage experiments, statistical analysis and constitutive modeling, J. Mech. Behav. Biomed. Mater., 2015, vol. 41, p. 92.
Sommer, G., Sherifova, S., Oberwalder, P.J., et al., Mechanical strength of aneurysmatic and dissected human thoracic aortas at different shear loading modes, J. Biomech., 2016, vol. 49, no. 12, p. 2374.
Shahmirzadi, D., Narayanan, P., Ronny, X.L., et al., Mapping the longitudinal wall stiffness heterogeneities within intact canine aortas using Pulse Wave Imaging (PWI) ex vivo, J. Biomech., 2013, vol. 46, no. 11, p. 1866.
Apostolakis, I.Z., McGarry, M.D., Bunting, E.A., et al., Pulse wave imaging using coherent compounding in a phantom and in vivo, Phys. Med. Biol., 2017, vol. 62, no. 5, p. 1700.
Danpinid, A., Luo, J., Vappou, J., et al., In vivo characterization of the aortic wall stress–strain relationship, Ultrasonics, 2010, vol. 50, no. 7, p. 654.
Chen, Q., Wang, Y., and Zhi-Yong, L., Re-examination of the mechanical anisotropy of porcine thoracic aorta by uniaxial tensile tests, BioMed. Eng. OnLine, 2016, vol. 15, no. 2, p. 167.
Schlicht, M.S., Khanafer, K., Duprey, A., et al., Experimental foundation for in vivo measurement of the elasticity of the aorta in computed tomography angiography, Eur. J. Vasc. Endovasc. Surg., 2013, vol. 46, no. 6, p. 447.
Valdez-Jasso, D., Banks, H.T., Haider, M.A., et al., Viscoelastic models for passive arterial wall dynamics, Adv. Appl. Math. Mech., 2009, vol. 1, no. 2, p. 151.
Cuomo, F., Roccabianca, S., Dillon-Murphy, D., et al., Effects of age-associated regional changes in aortic stiffness on human hemodynamics revealed by computational modeling, PLoS One, 2017, vol. 12, no. 3, p. 0173177
Khanafer, K., Schlicht, M.S., and Berguer, R., How should we measure and report elasticity in aortic tissue? Eur. J. Vasc. Endovasc. Surg., 2013, vol. 45, no. 4, p. 332.
ACKNOWLEDGMENTS
The study was supported by the Russian Science Foundation, project no. 16-15-00109.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Sherstyuk
Rights and permissions
About this article
Cite this article
Zhorzholiani, S.T., Talygin, E.A., Krasheninnikov, S.V. et al. Elasticity Change along the Aorta is a Mechanism for Supporting the Physiological Self-organization of Tornado-like Blood Flow. Hum Physiol 44, 532–540 (2018). https://doi.org/10.1134/S0362119718050171
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119718050171