Abstract
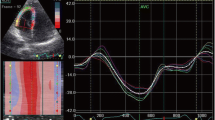
Chronic alcohol abuse not only leads to significant human psychic and social degradation, but also promotes the formation of alcoholic cardiomyopathy, which is one of the leading causes of high mortality of alcoholics. However, to date, there are no unified approaches in the prevention and treatment of alcoholic cardiomyopathy in clinics, primarily due to the lack of an adequate model in experimental pharmacology that could assess the stages of the formation of alcoholic cardiomyopathy objectively in real time, and thereby create the basis for the search and study of the mechanisms of action of drugs for the treatment of this serious disease. Studying the possibility of the use of echocardiography in experiments on rats with prolonged forced alcoholism is one of the approaches to solve this problem. It was shown that significant changes in intracardiac echocardiography hemodynamics corresponding to that known from the clinics begin to form from the 20th week of systematic consumption of alcohol by rats. By this time, the reduction in inotropic function of the heart in alcoholized rats compared to control rats is observed: the shortening fraction (SF) is 41.9% (40.3–42.2) and 51.3% (48.8–59.1), respectively, and the ejection fraction (EF) is 78.8% (77.4–79.2) and 87.5% (84.6–92.4), respectively, p ≈ 0.0215. The dilated heart failure develops in rats from the 24th week of regular alcohol consumption, as illustrated not only by the dynamic reduction of SF and EF, but also by the dilatation of the heart. For example, the end-systolic dimension of the left ventricle in animals consuming alcohol compared with the control rats more than doubled (4.31 mm (3.80–4.41) and 2.0 mm (1.85–2.36), p ≈ 0.0008, and the end-diastolic dimension was 5.95 mm (5.13–6.37) and 4.52 mm (3.85–4.90), respectively; p ≈ 0.0171. Thus, the echocardiographic picture characteristic of alcoholic dilated cardiomyopathy is formed by the end of the 24th week of chronic alcoholization.
Similar content being viewed by others
References
Leon, D.A., Shkolnikov, V.M., McKee, M., et al., Alcohol increases circulatory disease mortality in Russia: acute and chronic effects or misattribution of cause?, Int. J. Epidemiol, 2010, vol. 39, no. 5, p. 1279.
Rehm, J., Zatonski, W., Taylor, B., and Anderson, P., Epidemiology and alcohol policy in Europe, Addiction, 2011, vol. 106,suppl. 1, p. 11.
Vikhert, A.M. and Tsyplenkova, V.G., Alcoholic cardiomyopathy—risk factor of sudden death, Arkhiv Patol., 1984, vol. 46, no. 1, p. 14.
Kul’bitskii, B.N., Larev, Z.V., Fedulova, M.V., et al., Pathology of the cardiac conducting system in thanatogenesis of a sudden death at alcoholic cardiomyopathy and cardiac ishemic disease, Sud. Med. Ekspert., 2012, vol. 55, no. 2, p. 62.
Lang, R.M., Bierig, M., Devereux, R.B., et al., Recomendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Camber Quantification Writing Group, developed in conjunction with European Association of Echocardiography, a branch of the European Society of Cardiology, J. Am. Soc. Echocardiogr., 2005, vol. 18, p. 1440.
Martinez, P.F., Okoshi, K., Zornoff, L.A.M., et al., Ehocardiographic detection of congestive heart failure in postinfarction rats, J. Appl. Physiol., 2011, vol. 111, p. 543.
Khong, F.L., Zhang, Y., Edgley, A.J., et al., 3′,4′-dihydroxyflavonol antioxidant attenuates diastolic dysfunction and cardiac remodeling in streptozotocin-induced diabetic m(ren2)27 rats, PloS One, 2011, vol. 6, no. 7, p. e22777.
Leon, D.A., Shkolnikov, V.M., and McKee, M., Alcohol and Russian mortality: a continuing crisis, Addiction, 2009, vol. 104, no. 10, p. 1630.
Steel, G., Heart failure as result of alcoholism, Med. Chron., 1893, vol. 18, p. 1.
Shol’ts, D.M., Tsyplenkova, V.G., and Vikhert, A.M., Disturbances in the morfphology of the myocardium after a 20-week alcoholization and 6-week abstinence, Byull. Eksp. Biol. Med., 1989, vol. 108, no. 8, p. 244.
Kakturskii, L.V., Clinical morphology of alcoholic cardiomyopathy, Arkhiv Patol., 2009, vol. 71, no. 5, p. 21.
Preedy, V.R. and Richardson, P.J., Alcoholic cardiomyopathy: clinical and experimental pathological changes, Herz, 1996, vol. 21, no. 4, p. 241.
Jing, L., Li, W.W., Song, J., et al., Expression of tenascin-x in alcoholic cardiomyopathy and relation thereof to myocardial fibrosis: experimental with rats, Zhonghua Yi Xue Za Zhi, 2008, vol. 88, no. 36, p. 2570.
Iacovoni, A., De Maria, R., and Gavazzi, A., Alcoholic cardiomyopathy, J. Cardiovasc., Med. (Hagerstown), 2010, vol. 11, no. 12, p. 884.
Segel, L.D., The development of alcohol-induced cardiac dysfunction in the rat, Alcohol Alcoholosm, 1988, vol. 23, no. 5, p. 391.
Kim, S.D., Beck, J., Bieniarz, T., et al., A rodent model of alcoholic heart muscle disease and its evaluatiobn by echocardiography, Alcohol. Clin. Exp. Res., 2001, vol. 25, no. 3, p. 457.
Jing, L., Zhou, L., Zhang, F., et al., Tenascin-x facilitates myocardial fibrosis and cardiac remodeling through transforming factor-b1 and peroxisome proliferator-activated receptor g in alcoholic cardiomyopathy, Chin. Med. J., 2011, vol. 124, no. 3, p. 390.
Lang, C.H., Frost, R.A., Summer, A.D., and Vary, T.C., Molecular mechanisms responsible for alcohol-induced myopathy in skeletal muscle and heart, Int. J. Biochem. Cell Biol., 2005, vol. 37, p. 2180.
Ivashkin, V.T., Drapkina, O.M., and Ashikhmin, Ya., Problem of alcoholic cardiomyopathy, Klin. Med., 2006, no. 3, p. 11.
Tereshchenko, S.N., Zhirov, I.V., Kotaeva, E.A., and Malichenko, E.V., Alcoholic dilatational cardiomyopathy: Is the sign of equalty competent?, Kardiologiya, 2008, vol. 48, no. 3, p. 93.
Oba, T., Maeno, Y., Nagao, M., et al., Cellular redox state protects acetaldehyde-induced alteration in cardiomyocyte by modifying Ca2+ release from sarcoplasmic reticulum, Am. J. Physiol. Heart Circ. Physiol., 2008, vol. 294, no. 1, p. H121.
Ren, J. and Wold, L.E., Mechanisms of alcoholic heart disease, Ther. Adv. Cardiovasc. Dis., 2008, vol. 2, no. 6, p. 497.
Thomas, A.P., Rozanski, D.J., Renard, D.C., et al., Effects of ethanol on the contractile function of the heart: a review, Alcohol. Clin. Exp. Res., 1994, vol. 18, p. 121.
Swynghedauw, B., Molecular mechanisms of myocardial remodeling, Physiol. Rev., 1999, vol. 79, p. 215.
Piano, M.R., Kim, S.D., and Jarvis, C., Cellular targets linked to cardiac remodeling in heart failure: targets for pharmacologic intervention, J. Cardiovasc. Nurs., 2000, vol. 14, p. 1.
Correale, M., Laonigro, I., Altomare, E., and Biase, M., Alcohol-induced cardiac disease, G. Ital. Cardiol., (Rome), 2009, vol. 10, no. 1, p. 18.
Silvestri, F. and Bussani, R., Hypoxic right ventricular cardiomyopathy. a morphological and pathogenetic study on the myocardial atrophy and fatty infiltration, Pathologica, 1990, vol. 82, no. 1082, p. 593.
Jing, L., Zhou, L.J., Li, W.M., et al., Carnitne regulates myocardial metabolism by peroxisome proliferator-activated receptor-alpha (PPARlpha) in alcoholic cardiomyopathy, Med. Sci. Monit., 2011, vol. 17, no. 1, p. BR1–9.
Klein, H.H., Spaar, U., and Kreuzer, H., The effect of chronic ethanol consumption on enzyme activities of the energy-supplying metabolism and the alcoholaldehyde oxidizing system in rat hearts, Basic Res. Cardiol., 1984, vol. 79, no. 2, p. 238.
Auffermann, W., Buser, P., Wu, S., et al., Activation of glycolysis with isoproterenol but not digoxin reverses chronic alcohol depression in hamster hearts, Alcohol. Clin. Exp. Res., 1992, vol. 16, no. 3, p. 505.
Author information
Authors and Affiliations
Additional information
Original Russian Text © S.A. Kryzhanovsky, L.G. Kolik, I.B. Tsorin, E.O. Ionova, V.N. Stolyaruk, M.B. Vititnova, A.V. Nadorova, S.B. Seredenin, 2014, published in Fiziologiya Cheloveka, 2014, Vol. 40, No. 1, pp. 122–128.
Rights and permissions
About this article
Cite this article
Kryzhanovsky, S.A., Kolik, L.G., Tsorin, I.B. et al. On the potential use of echocardiography for assessing the formation of alcoholic cardiomyopathy. Hum Physiol 40, 105–110 (2014). https://doi.org/10.1134/S0362119714010101
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119714010101