Abstract
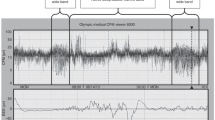
Data on the formation of cyclic sleep organization and melatonin production in full-term newborn infants with intrauterine growth retardation (IUGR) are presented. It has been shown that disordered cyclic sleep organization in IUGR newborns indicates the degree of intrauterine brain damage as a result of chronic hypoxia. The absence of cyclic sleep organization, the disordered formation of the paradoxical phase combined with delayed development of tonic, congenital reflex reactions, and low melatonin production give evidence of severe brain damage, which warrants combined therapy in the early neonatal period of life.
Similar content being viewed by others
References
Evsyukova, I.I., Fomenko, B.A., Andreeva, A.A., et al., Features of adaptation of newborn infants with intrauterine growth retardation, Zh. Akush. Zhen. Boleznei, 2003, vol. 52, no. 4, p. 23.
Deryugina, O.A., Features of neonatal adaptation of preterm newborns with IUGR and correction of its disorders, Cand. Sci. (Med.) Dissertation, Moscow, 1990.
Dessi, A., Ottonello, G., and Fanos, V., Physiopathology of intrauterine growth retardation: from classical data to metabolomics, J. Matern. Fetal Neonatal Med., 2012, vol. 25, Suppl. 5, p. 13.
Shavit, T., Ashual, E., Regev, R., et al., Is it necessary to induce labor in cases of intrauterine growth retardation at term? J. Perinat. Med., 2012, vol. 40, no. 5, p. 539.
Barashnev, Yu.I., Hypoxic encephalopathy: hypotheses of the pathogenesis of cerebral disorders and the search for methods of medicinal therapy, Ross. Vestn. Perinat. Pediatr., 2002, vol. 47, no. 1, p. 6.
Pal’chik, A.B., Vvedenie v nevrologiyu razvitiya (Introduction to Developmental Neurology), St. Petersburg: Kosta, 2007.
Evsyukova, I.I., Formation of the CNS functions and the pathogenesis of disorders under unfavorable conditions of intrauterine fetal life, Vestn. Ross. Assotsiat. Akush. Ginekol., 1997, no. 3, p. 31.
Lapillonne, A., Intrauterine growth retardation and adult outcome, Bull. Acad. Natl. Med., 2011, vol. 195, no. 3, p. 477.
Barker, D.J.P., Eriksson, J.G., Forsen, T., and Osmond, C., Fetal origins of adult disease: strength of effects and biological basis, Int. J. Epidemiol., 2002, vol. 31, no. 6, p. 1235.
Wake, M., Morton-Allen, E., Poulakis, Z., et al., Prevalence, stability, and outcomes of cry-fuss and sleep problems in the first two years of life: prospective community-based study, Pediatrics, 2006, vol. 117, no. 3, p. 836.
Geib, L.T. and Nunes, M.L., The incidence of sudden death syndrome in a cohort of infants, J. Pediatr., 2006, vol. 82, no. 1, p. 21.
Moore, M., Allison, D., and Rosen, C.L., A review of pediatric nonrespiratory sleep disorders, Chest, 2006, vol. 130, no. 4, p. 1252.
Kel’manson, I.A., Natural course of sleep disorders in infants in the first year of life, in Aktual’nye problemy somnologii (Topical Somnology Problems) (Proc. VII All-Russian Conf.), Moscow, 2010, p. 32.
Santos, I.S., Mota, D.M., and Matijasevich, A., Epidemiology of co-sleeping and nighttime waking at 12 months in a birth cohort, J. Pediatr., 2008, vol. 84, no. 2, p. 114.
Danker-Hopfe, H., Growth and development of children with a special focus on sleep, Prog. Biophys. Mol. Biol., 2011, vol. 107, no. 3, p. 333.
Pearl, P.L., Childhood sleep disorders: diagnostic and therapeutic approaches, Curr. Neurol. Neurosci. Rep., 2002, vol. 2, no. 2, p. 150.
Nixon, G.M., Thompson, J.M.D., Han, D.Y., et al., Short sleep duration in middle childhood: risk factors and consequences, Sleep, 2008, vol. 31, no. 1, p. 71.
Fallone, G., Acebo, C., Arnedt, J.T., et al., Effects of active sleep restriction on behavior, sustained attention, and response inhibition in children, Percep. Mot. Skills, 2001, vol. 93, no. 1, p. 213.
Hemmi, M.H., Wolke, D., and Schneider, S., Associations between problems with crying, sleeping, and/or feeding in infancy and long-term behavioral outcomes in childhood: a meta-analysis, Arch. Dis. Child., 2011, vol. 96, no. 7, p. 622.
Gottlieb, D.J., Chase, C., Vezina, R.M., et al., Sleepdisordered breathing symptoms are associated with poorer cognitive function in 5-year-old children, J. Pediatr., 2004, vol. 145, no. 4, p. 458.
Wolke, D., Rizzo, P., and Woods, S., Persistent infant crying and hyperactivity problems in middle childhood, Pediatrics, 2002, vol. 109, no. 6, p. 1054.
Dominguez-Ortega, L. and de Vicente-Colomina, A., Attention deficit-hyperactivity disorder and sleep disorders, Med. Clin. (Barc.), 2006, vol. 126, no. 13, p. 500.
Evsyukova, I.I., Formation of the mechanisms of regulation of the rhythm of cardiac activity and respiration in the sleep cycle of newborns under different conditions of intrauterine growth, Doctoral (Med.) Dissertation, Leningrad, 1983, p. 42.
Finkel’, M.L., Influence of conditions of normal and late toxicosis-complicated pregnancy on the establishment of the external respiration function in newborn infants, Cand. Sci. (Med.) Dissertation, Leningrad, 1975.
Shevchenko, O.T., State of cerebral circulation in the sleep cycle of healthy and hypoxia-afflicted neonates, Cand. Sci. (Med.) Dissertation, Leningrad, 1986.
Batilova, T.V., State of central hemodynamics in the sleep cycle in newborn infants under different conditions of intrauterine life, Cand. Sci. (Med.) Dissertation, Leningrad, 1989.
Kosov, M.N., Features of capnogram in newborn infants under normal and unfavorable intrauterine development, Cand. Sci. (Med.) Dissertation, St. Petersburg, 1999.
Shepoval’nikov, A.N., Aktivnost’ spyashchego mozga. Elektropoligraficheskoe issledovanie fiziologicheskogo sna u detei (The Activity of the Sleeping Brain. Electropolygraphic Study of the Physiological Sleep in Children), Leningrad: Nauka, 1971.
Evsyukova, I.I. and Polyakova, G.P., Sleep of newborns as an indicator of the functional state of the central nervous system, Akush. Ginekol., 1979, no. 9, p. 58.
Dan, B. and Boyd, S.G., A neurophysiological perspective on sleep and its maturation, Dev. Med. Clin. Neurol., 2006, vol. 48, no. 9, p. 773.
Vanhatalo, S. and Kaila, K., Development of neonatal EEG activity: from phenomenology to physiology, Semin. Fetal Neonatal Med., 2006, vol. 11, no. 6, p. 471.
Scher, M.S., Neonatal encephalopathies as classified by EEG-sleep criteria: severity and timing based on clinical/pathologic correlations, Pediatr. Neurol., 1994, vol. 11, no. 3, p. 189.
Lamblin, M.D., Andre, M., Auzoux, M., et al., Indications of electroencephalogram in the newborn, Arch. Pediatr., 2004, vol. 11, no. 7, p. 829.
Scher, M.S., Ontogeny of EEG-sleep from neonatal through infancy periods, Sleep Med., 2008, vol. 9, no. 6, p. 615.
Stephan-Blanchard, E., Telliez, F., Leke, A., et al., The influence of in utero exposure to smoking on sleep patterns in preterm neonates, Sleep, 2008, vol. 31, no. 12, p. 1683.
Bespyatykh, A.Yu., Brodskii, V.Ya., Burlakova, O.V., et al., Melatonin: teoriya i praktika (Melatonin: Theory and Practice), Rapoport, S.I. and Golichenkov, V.A., Eds., Moscow: Medpraktika-M, 2009.
Rodriguez, C., Mayo, J.C., Sainz, R.M., et al., Regulation of antioxidant enzymes: a significant role for melatonin, J. Pineal Res., 2004, vol. 36, no. 1, p. 1.
Mayo, J.C., Sainz, R.M., Antoli, I., et al., Melatonin regulation of antioxidant enzyme gene expression, Cell Mol. Life Sci., 2002, vol. 59, no. 10, p. 1706.
Tan, D.X., Manchester, L.C., Terron, M.P., et al., One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species, J. Pineal Res., 2007, vol. 42, no. 1, p. 28.
Munoz-Hoyos, A., Bonillo-Perales, A., Avila-Villegas, R., et al., Melatonin levels during the first week of life and their relation with the antioxidant response in perinatal period, Neonatology, 2007, vol. 92, no. 3, p. 209.
Fomenko, B.A., Influence of the intrauterine growth conditions on the formation of perinatal CNS pathology in preterm infants, Cand. Sci. (Med.) Dissertation, St. Petersburg, 1995, p. 19.
Pauer, M.L. and Shul’kin, J., Rozhdenie rebenka, distress i risk boleznei (Childbirth, Distress, and the Risk of Diseases), Moscow: Triada-X, 2010.
Gogfrey, K.M., The role of the placenta in fetal programming, Placenta, 2002, vol. 23,Suppl. A, p. 20.
Rees, S., Harding, R., and Walker, D., The biological basis of injury and neuroprotection in the fetal and neonatal brain, Int. J. Dev. Neurosci., 2011, vol. 29, no. 6, p. 551.
Galland, B.C., Taylor, B.J., Bolton, D.P., and Sayers, R.M., Heart rate variability and cardiac reflexes in small for gestational age infants, J. Appl. Physiol., 2006, vol. 100, no. 3, p. 933.
Barker, D.J.P., The development origins of adult disease, J. Am. College Nutrition, 2004, vol. 23,Suppl. 6, p. 588.
Jansson, T. and Powell, T., Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches, Clin. Sci., 2007, vol. 113, no. 1, p. 1.
Luk’yanova, L.D., Mitochondrial dysfunction is a type pathological process, the molecular mechanism of hypoxia, in Problemy gipoksii: molekulyarnye, fiziologicheskie i meditsinskie aspekty (Problems of Hypoxia: The Molecular, Physiological, and Medical Aspects), Luk’yanova, L.D. and Ushakov, I.B., Eds., Moscow, 2004.
Luk’yanova, L.D., Modern problems of adaptation to hypoxia. The signal mechanisms and their role in systemic regulation, Patol. Fiziol. Eksp. Ter., 2011, no. 1, p. 2.
Kostyuk, P.G., Stanika, R.I., and Luk’yanets, E.A., Intracellular mechanisms of hypoxic disorders of the neural cell function, in Problemy gipoksii: molekulyarnye, fiziologicheskie i meditsinskie aspekty (Problems of Hypoxia: The Molecular, Physiological, and Medical Aspects), Luk’yanova, L.D. and Ushakov, I.B., Eds., Moscow, 2004.
Rees, S., Harding, R., and Walker, D., An adverse intrauterine environment: implications or injury and altered development of the brain, Int. J. Dev. Neurosci., 2008, vol. 26, no. 1, p. 3.
Hossain, M.A., Hypoxic-ischemic injury in neonatal brain: involvement of a novel neonatal molecule in neuronal cell death and potential target for neuroprotection, Int. J. Dev. Neurosci., 2008, vol. 26, no. 1, p. 93.
Mallard, C., Loeliger, M., Copolov, D., and Rees, S., Reduced number of neurons in the hippocampus and the cerebellum in the postnatal guinea-pig following growth restriction, Neuroscience, 2000, vol. 100, no. 2, p. 327.
Raman, L., Take, I., Ennis, K., et al., In vivo effect of chronic hypoxia on the neurochemical profile of the developing rat hippocampus, Brain Res. Dev., 2005, vol. 156, no. 2, p. 202.
Jensen, A., Garnier, Y., Middelanis, J., and Berger, R., Perinatal brain damage: from pathophysiology to prevention, Eur. J. Obstet. Gynecol. Reprod. Biol., 2003, vol. 110, Suppl. 1, p. 70.
Berger, R., Garnier, Y., and Jensen, A., Perinatal brain damage: underlying mechanisms and neuroprotective strategies, J. Soc. Gynecol. Investig., 2002, vol. 9, no. 6, p. 319.
Dieni, S. and Rees, S., Dendritic morphology is altered in hippocampal neurons following prenatal compromise, J. Neurobiol., 2003, vol. 55, no. 1, p. 41.
Rees, S., Mallard, C., Breen, S., et al., Fetal brain injury following prolonged hypoxemia and placental insufficiency: a review, Comp. Biochem. Physiol. Mol. Integr. Physiol., 1998, vol. 119, no. 3, p. 653.
Zakharova, E.I., Svinov, M.M., Germanova, E.N., et al., Mechanisms of involvement of cholinergic systems in the processes of morphofunctional reorganization of the neocortex and hippocampus under brain hypoxia conditions, in Problemy gipoksii: molekulyarnye, fiziologicheskie i meditsinskie aspekty (Problems of Hypoxia: The Molecular, Physiological, and Medical Aspects), Luk’yanova, L.D. and Ushakov, I.B., Eds., Moscow, 2004.
Aloe, F., Pinto de Azevedo, A., and Hasan, R., Sleepwake cycle mechanisms, Rev. Bras. Psiquiatr., 2005, vol. 27,suppl. 1, p. 33.
Fan, X. and van Bel, F., Pharmacological neuroprotection after perinatal asphyxia, J. Matern. Fetal Neonatal Med., 2010, vol. 23,Suppl. 3, p. 17.
Ozdemir, O.M.A., Ergin, H., and Sahiner, T., Electrophysiological assessment of the brain function in term SGA infants, Brain Res., 2009, vol. 1270, no. 3, p. 33.
Jan, J.E., Reiter, R.J., Wasdell, M.B., and Bax, M., The role of the thalamus in sleep, pineal melatonin production, and circadian rhythm sleep disorders, J. Pineal Res., 2009, vol. 46, no. 2, p. 1.
Ferber, S.G., Als, H., McAnulty, G., et al., Melatonin and mental capacities in newborn infants, J. Pediatr., 2011, vol. 159, no. 1, p. 99.
Gitto, E., Pellegrino, S., Gitto, P., et al., Oxidative stress of the newborn in the pre- and postnatal period and the clinical utility of melatonin, J. Pineal Res., 2009, vol. 46, no. 2, p. 128.
Wakatsuki, A., Okatani, Y., and Kaneda, C., Melatonin protects fetal rat brain against oxidative mitochondrial damage, J. Pineal Res., 2001, vol. 30, no. 1, p. 22.
Liu, J., Somera-Molina, K.C., Hudson, R.L., and Dubocovich, M.L., Melatonin potentiates running wheel-induced neurogenesis in the dentate gyrus of adult C3H/HeN mice hippocampus, J. Pineal Res., 2013, vol. 54, no. 2, p. 222.
Tauman, R., Zisapel, N., Laudon, M., et al., Melatonin production in infants: association with perinatal factors and development, Pediatr. Neurol., 2002, vol. 26, no. 5, p. 379.
Kennaway, D.J., Stamp, G.E., and Goble, F.C., Development of melatonin production in infants and the impact of prematurity, J. Clin. Endocrinol. Metab., 1992, vol. 75, no. 2, p. 367.
Kennaway, D.J., Lushington, K., Dawson, D., et al., Urinary 6-sulfatoxymelatonin excretion and aging: new results and a critical review of the literature, J. Pineal Res., 1999, vol. 27, no. 4, p. 210.
Caldelas, J., Tejadilla, D., Gonzalez, B., et al., Diurnal pattern of clock gene expression in the hypothalamus of the newborn rabbit, Neuroscience, 2007, vol. 144, no. 2, p. 395.
Tret’yakova, M.B., Melatonin and thrombocyte functional activity in newborn infants with intrauterine growth retardation, Cand. Sci. (Med.) Dissertation, St. Petersburg, 2006.
Chen, Y.-C., Tain, Y.-L., Sheen, J.-M., and Huang, L.-T., Melatonin utility in neonates and children, J. Form. Med. Associat., 2012, vol. 111, p. 57.
Szymusiak, R., Gvilia, I., and McGinty, D., Hypothalamic control sleep, Sleep, 2008, vol. 8, no. 4, p. 291.
Sanduk, R., Melatonin and maturation of REM sleep, Int. J. Neurosci., 1992, vol. 63, nos. 1–2, p. 105.
Alonso-Alconada, D., Alvarez, A., Lacalle, J., and Hilario, E., Histological study of the protective effect of melatonin on neural cells after neonatal hypoxiaischemia, Histol. Histopathol., 2012, vol. 27, no. 6, p. 771.
Ozyener, F., Cetinkaya, M., Alkan, T., et al., Neuroprotective effects of melatonin administered alone or in combination with topiramate in neonatal hypoxicischemic rat model, Restor. Neurol. Neurosci., 2012, vol. 30, no. 5, p. 435.
Villapol, S., Fau, S., Renolleau, S., et al., Melatonin promotes myelination by decreasing white matter inflammation after neonatal stroke, Pediatr. Res., 2011, vol. 69, no. 1, p. 51.
Lekic, T., Manaenko, A., and Rolland, W., Neuroprotection by melatonin after germinal matrix hemorrhage in neonatal rats, Acta Neurochir. Suppl., 2011, vol. 111, p. 201.
Manjarrez, G., Cisneros, I., Herrera, R., et al., Prenatal impairment of brain serotonergic transmission in infants, J. Pediatr., 2005, vol. 147, no. 5, p. 592.
Hernandez-Rodriquez, J., Meneses, L., Herera, R., and Manjarrez, G., Another abnormal trait in the serotonin metabolism path in intrauterine growthrestricted infants, Neonatology, 2008, vol. 95, no. 2, p. 125.
Riese, M.L., Size for gestational age and neonatal sleep variables: behavioral indices of risk in full-term twins, Acta Genet. Med. Gemellol., 1993, vol. 42, no. 1, p. 23.
Curzi-Dascalova, L., Peirano, P., and Christova, E., Respiratory characteristics during sleep in healthy small-for-gestational-age newborns, Pediatrics, 1996, vol. 97, no. 4, p. 554.
Author information
Authors and Affiliations
Additional information
Original Russian Text © I.I. Evsyukova, O.V. Koval’chuk-Kovalevskaya, N.A. Maslyanyuk, D.S. Dodkhoev, 2013, published in Fiziologiya Cheloveka, 2013, Vol. 39, No. 6, pp. 63–71.
Rights and permissions
About this article
Cite this article
Evsyukova, I.I., Koval’chuk-Kovalevskaya, O.V., Maslyanyuk, N.A. et al. Features of cyclic sleep organization and melatonin production in full-term newborns with intrauterine growth retardation. Hum Physiol 39, 617–624 (2013). https://doi.org/10.1134/S0362119713060030
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0362119713060030