Abstract
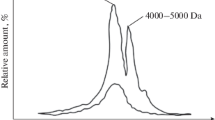
The potentialities of using ultrafiltration, nanofiltration, and reverse-osmosis membranes for removing organic compounds from water are considered. Experimental results obtained by the authors and the information taken from the literature characterizing the interaction between membranes and organic compounds contained in natural water are presented. Data are obtained concerning the efficiency of different membrane technologies in the removal of organic compounds from natural water. The growth rate of the deposits of organic substances having different molecular weight has been studied. The effect of the membrane’s material and the hydrodynamic mode of the membrane’s operation on the formation rate of organic deposits has been demonstrated. The influence of these substances’ nature, their concentration in natural water, and the molecular weight thereof on the deposition rate on membranes have been revealed. In the experiments, tap water taken from the Moscow water supply and river water were used. The results of the authors’ experiments and the results obtained by other Russian and foreign researchers presented in the paper make it possible to consider ultrafiltration as a reliable way to retain organic macromolecules as well as finely dispersed and colloidal impurities. With retaining low-molecular organic compounds that determine the color of water, ultrafiltration is restricted. In the synthesis of nanofiltration membranes, films with different pore size and charge are used. Therefore, for a specific water source and water consumer, an individual choice of nanofiltration membranes is required, a wide range of which is available in the market. Nanofiltration membranes of the NE-90 and NE-70 types for surface waters having a color of 20–100 degrees provide a 85 and 75% decrease in the oxidability level, respectively. According to the results of studies concerning the efficiency of artesian water purification, domestic OPMN-K nanofiltration membranes can also profoundly purify water from natural organic compounds. Among all the membranes used in the water treatment technology, reverse-osmosis membranes have the smallest pore size; their use provides retaining organic compounds with a molecular weight lower than 100 Da. The studies have shown that reverse-osmosis membranes exhibit a 99.5% retention level for the compounds with a molecular weight of 150 Da and a 99.9% retention level for the compounds with a molecular weight higher than 1000 Da. To reveal the nature and concentration of organic compounds deposited on the membranes, water spectrograms have been used.









Similar content being viewed by others
REFERENCES
A. I. Schafer, A. G. Fane, and T. D. Waite, “Cost factors and chemical pretreatment effects in the membrane filtration of waters containing natural organic matter,” Water Resour. 35, 1509–1517 (2001).
A. N. Samodurov, S. E. Lysenko, S. L. Gromov, A. A. Panteleev, and E. B. Fedoseeva, “The use of reverse osmosis technology for water treatment in power engineering,” Therm. Eng. 53, 439–443 (2006).
G. V. Slavinskaya and O. V. Kurenkova, “Change of physicochemical and technological characteristics of ion-exchange materials in natural water conditioning units,” Sorbtsionnye Khromatogr. Protsessy 13, 322–331 (2013).
L. K. Goncharova and N. A. Strebkova, “Results obtained from tests of organics-absorbing anion-exchange resins for the water treatment plant at the Ivanovo district power station,” Therm. Eng. 55, 351–354 (2008).
E. Aoustin, A. I. Schäfer, A. G. Fane, and T. D. Waite, “Ultrafiltration of natural organic matter,” Sep. Purif. Technol. 22–23, 63–78 (2001).
S. L. Gromov, M. P. Kovalev, S. E. Lysenko, A. A. Panteleev, A. N. Samodurov, and A. R. Sidorov, “The use of modern integrated membrane technologies to improve the quality of feed water in energy enterprises,” Vodopodgot. Vodoochistka. Vodosnabzh., No. 2, 20–29 (2008).
E. B. Yurchevskii, A. G. Pervov, and M. A. Pichugina, “Water purification from organic impurities using membrane water treatment technologies,” Energosberezhenie Vodopodgot., No. 5, 32–45 (2016).
V. Mavrov, H. Chmiel, J. Kluth, J. Meier, F. Heinrich, P. Ames, K. Backes, and P. Usner, “Comparative study of different MF and UF membranes for drinking water production,” Desalination 117, 189–196 (1998).
L. Song, “Flux decline in crossflow microfiltration and ultrafiltration: Mechanisms and modeling of membrane fouling,” J. Membrane Sci. 139, 183–200 (1998).
J.-M. Laine, D. Vial, and P. Moulart, “Status after 10 years of operation — overview of UF technology today,” in Proc. Conf. on Membranes in Drinking and Industrial Water Production, Paris, France, Oct. 3–6,2000 (Elsevier, Amsterdam, 2000), Vol. 1, pp. 412–428.
E. B. Yurchevskii, A. G. Pervov, and A. P. Andrianov, “Studying the process of formation of sediments of suspended, colloidal, organic and crystalline substances on the surface of membranes and ways to increase the life of membrane systems before chemical washing,” Energosberezhenie Vodopodgot., No. 3, 3–8 (2006).
G. G. Kagramanov, Diffusion Membrane Processes (Ross. Khim.-Tekhnol. Univ. im. D. I. Mendeleeva, Moscow, 2009) [in Russian].
Yu. I. Dytnerskii, Baromembrane Processes (Khimiya, Moscow, 1986) [in Russian].
A. G. Pervov, “A simplified RO process design based on understanding of fouling mechanisms,” Desalination 126, 227–247 (1999).
R. Bian, K. Yamamoto, and Y. Watanabe, “The effect of shear rate on controlling the concentration polarization and membrane fouling,” in Proc. Conf. on Membranes in Drinking and Industrial Water Production, Paris, France, Oct. 3–6,2000 (Elsevier, Amsterdam, 2000), Vol. 1, pp. 421–432.
M. Futselaar, H. Schonewille, and W. van der Meer, “Direct capillary nanofiltration for surface water,” Desalination 157, 135–136 (2003).
M. Thanuttamavong, J. I. Oh, K. Yamamoto, and T. Urase, “Comparison between rejection characteristics of natural organic matters and inorganic salts in ultralow pressure nanofiltration for drinking water production,” in Proc. Conf. on Membranes in Drinking and Industrial Water Production, Paris, France, Oct. 3–6,2000 (Elsevier, Amsterdam, 2000), Vol. 1, pp. 269–282.
A. G. Pervov, A. P. Andrianov, D. V. Spitsov, and E. B. Yurchevskii, “The effect of the properties of membranes and membrane channel designs on the intensity of sedimentation processes and decrease in productivity,” Membrany, No. 2, 3–14 (2010).
I. Champlin Iory, “Using circulation tests to model natural organic matter adsorption and particle deposition by spiral-wound nanofiltration membrane elements,” in Proc. Conf. on Membranes in Drinking and Industrial Water Production, Paris, France, Oct. 3–6,2000 (Elsevier, Amsterdam, 2000), Vol. 1, pp. 155–165.
A. P. Vertinskii, “The use of spectrophotometric method for monitoring natural waters,” Usp. Sovrem. Estestvozn., No. 5. Ch. 1, 205–207 (2014).
Yu Dan Su, A. G. Pervov, and X. Quyet Nguyen, “Formation of organic deposits on low-pressure reverse osmotic membranes in treating water from surface sources,” Vestn. MGSU 14, 1180–1195 (2019).
A. G. Pervov, G. Ya. Rudakova, and R. V. Efremov, “Development of programs for technological calculation of reverse osmosis and nanofiltration systems using “Aminat” reagents,” Vodosnabzh. Sanit. Tekh., No. 7, 21–28 (2009).
A. G. Pervov, A. P. Andrianov, D. V. Spitsov, and L. V. Rudakova, “New technologies and devices based on ultra- and nanofiltration methods for water supply and heat supply systems,” Vodosnabzh. Sanit. Tekh., No. 7, 12–19 (2009).
A. I. Shafer, A. G. Fane, and T. D. Waite, “Fouling effects on rejection in the membrane filtration of natural waters,” in Proc. Conf. on Membranes in Drinking and Industrial Water Production, Paris, France, Oct. 3–6,2000 (Elsevier, Amsterdam, 2000), Vol. 2, pp. 257–274.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Polyakov
Rights and permissions
About this article
Cite this article
Jurchevsky, E.B., Pervov, A.G. Potentialities of Membrane Water Treatment for Removing Organic Pollutants from Natural Water. Therm. Eng. 67, 484–491 (2020). https://doi.org/10.1134/S0040601520070095
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040601520070095