Abstract
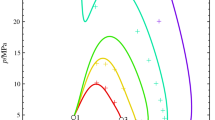
Results from a detailed study are presented in which we analyzed the shape of critical lines of binary aqueous solutions like H2O + common salt and H2O + hydrocarbon. We also present the results from a comparative analysis of data on the critical parameters of such solutions reported in the literature. The critical lines of the solutions are analyzed in different projections. Accuracy, reliability, and consistency of the data considered are estimated, and recommendations on using them for scientific and practical purposes are given.
Similar content being viewed by others
References
I. R. Krichevskii, “The Thermodynamics of Critical Phenomena in Infinitely Diluted Binary Solutions,” Zh. Fiz. Khim. 41, 1332–1343 (1967).
P. H. von Konynenburg and P. L. Scott, “Critical Lines and Phase Equilibria in Binary Van der Waals Mixtures,” Philos. Trans. Roy. Soc. London A. 298, 495–540 (1980).
D. S. Tsyklis and L. A. Rott, “Phase Equilibrium between Two Immiscible Gaseous Phases,” J. Chem. Rev., 351–361 (1967).
A. A. Povodyrev, M. A. Anisimov, J. V. Senders, et al., Evaluation of the Critical Locus of Aqueous Solutions of Sodium Chloride, Technical Report to IAPWS, IPST (Univ. of Marylant at College Park, 1997).
A. A. Povodyrev, M. A. Anisimov, J. V. Senders, et al., Guideline on Critical Locus of Aqueous Solutions of Sodium Chloride (IAPWS, Prague, 2000).
I. Kh. Khaibullin, B. Novikov, A. M. Copeliovich, and A. M. Besedin, “Phase Diagrams for Steam Solutions and Caloric Properties of Two-and Three-Component Systems: H2O + NaCl, H2O + Na2SO4, H2O + NaCl + Na2SO4,” in Water and Steam, Ed. by J. Straub and K. Scheffler (Pergamon Press, New York, 1979), pp. 641–647.
J. L. Bischoff, R. J. Rosenbauer, and K. S. Pitzer, “The System NaCl-H2O: Relations of Vapor-Liquid Near the Critical Temperature of Water and of the Vapor-Liquid-Halite from 300 to 500°C,” Geochim.et Cosmochim Acta. 50, 1437–1444 (1986).
K. S. Pitzer, J. L. Bischoff, and R. J. Rosenbauer, “Critical Behavior of Dilute NaCl in H2O,” Chem. Phys. Lett. 134, 30–63 (1986).
W. L. Marshall and E. V. Jones, “Liquid-Vapor Critical Temperatures of Aqueous Electrolyte Solutions,” J. Inorg. Nucl. Chem. 36, 2313–2318 (1974).
A. I. Abdulagatov, G. V. Stepanov, and I. M. Abdulagatov, “Critical Properties of Aqueous Solutions. Part I. Experimental Data,” Teploenergetika, No. 8, 72–78 (2008) [Therm. Eng., No. 8 (2008)].
A. I. Abdulagatov, G. V. Stepanov, and I. M. Abdulagatov, “Asymptotic Behaviors of the Thermodynamic Properties of Infinitely Diluted Aqueous Solutions Near the Critical Point of Pure Water,” in Ultrasound and Thermodynamic Properties of Substances (KGU, Kursk, 2007).
A. I. Viktorov, A. Fredenslund, and N. A. Smirnova, “Fluid Phase Equilibria in Water: Natural Gas Component Mixtures and Their Description by the Whole Group-Contribution Equation of State,” Fluid Phase Equilib. 66, 187–210 (1991).
J. F. Connolly, “Solubility of Hydrocarbons in Water Near the Critical Temperature,” J. Chem. Eng. Data 11, 13–17 (1966).
J. G. Roof, “Three-Phase Critical Point in Hydrocarbon-Water Systems,” J. Chem. Eng. Data 15, 301–303 (1970).
Z. Alwani and G. M. Schneider, “Phase Equilibrium, Critical Phenomena, and pVT Data in Binary Mixtures of Water with Aromatic Hydrocarbons up to 2200 bar and 420°C,” Ber. Bunsenges. Phys. Chem. 73, 294–301 (1967).
A. L. Horvath, “References Literature to the Critical Properties of Aqueous Electrolyte Solutions,” J. Chem. Inf. Comput. Sci. 15, 245 (1975).
V. M. Valyashko, “Studies of Water-Salt Systems at Elevated Temperatures and Pressures,” Ber. Bunsenges. Phys. Chem. 81, 388 (1977).
V. M. Valyashko, “Phase Behavior in Binary and Ternary Water-Salt Systems at High Temperatures and Pressures,” Pure and Appl. Chem. 69, 2271 (1997).
C. J. Barton, G. M. Hebert, and W. L. Marshall, “Aqueous Systems at High Temperature — II: Liquid-Liquid Immiscibility in the System UO3-SO3-N2O5-H2O above 300°C,” J. Inorg. Nucl. Chem. 21, 141–151 (1961).
W. L. Marshall, E. V. Jones, G. M. Hebert, and F. J. Smith, “Aqueous Systems at High Temperature-VII: Liquid-Liquid Immiscibility and Critical Phenomena in the Systems UO3-SO3-H2O, UO3-SO3-D2O, and CuO-SO3-D2), 270–430°C,” J. Inorg. Nucl. Chem. 24, 995–1000 (1962).
E. V. Jones and W. L. Marshall, “Aqueous Systems at High Temperature — XIII: Investigations on the System UO3-Li2O-SO3-D2O; Liquid-Liquid Immiscibility and Critical Phenomena, 300–410°C,” J. Inorg. Nucl. Chem. 26, 281–285 (1964).
W. L. Marshall, J. S. Gill, and R. Slusher, “Aqueous Systems at High Temperature — VI: Investigations on the System NiO-SO3-H2O and Its D2O Analogue from 10−4 to 3 m SO3, 150–450°C, J. Inorg. Nucl. Chem. 24, 889–897 (1962).
W. L. Marshall, R. Slusher, and F. J. Smith, “Aqueous Systems at High Temperature — IX: Investigations on the System Li2SO4-H2SO4-H2O and its D2O analogue, 200–470°C: Solubilities and Critical Phenomena,” J. Inorg. Nucl. Chem. 25, 559–566 (1963).
J. L. Bischoff and K. S. Pitzer, “Phase Relations and Adiabats in Boiling Seafloor Geothermal Systems,” Earth and Planetary Sci. Letters 75, 327–338 (1985).
A. I. Abdulagatov, G. V. Stepanov, and I. M. Abdulagatov, “Crossover Equation of State and Microstructure Properties of the Infinite-Dilute Solutions Near the Critical Point of the Pure Solvent,” Russ. J. Structural Chem. 42, 585 (2001).
R. W. Potter, R. S. Babcock, and G. K. Czamanske, “An Investigation of the Critical Liquid-Vapor Properties of Dilute KCl Solutions,” J. Solution Chem. 5, 223–230 (1976).
W. Wagner and A. Pruss, “The IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use,” J. Phys. Chem. Ref. Data 31, 387–535 (2002).
R. Span and W. Wagner, “Equation of State for Technical Applications II. Results for Nonpolar Fluids,” Int. J. Thermophys. 24, 41–109 (2003).
F.-Y. Jou and A. E. Mather, “Vapor-Liquid-Liquid Locus of the System Pentane + Water,” J. Chem. Eng. Data 45, 728–729 (2000).
P. C. Gillespie and G. M. Wilson, Vapor-Liquid and Liquid-Liquid Equilibria: Water-Methane, Water-Carbon Dioxide, Water-Hydrogen Sulfide, Water-n-Pentane, Water-Methane-n-Pentane, Gas Processors Association Research Report RR-48, Project 758-B-77 (Wiltec Res. Co., Inc., Provo, Utah, 1982).
C. Tsonopoulos and G. M. Wilson, “High-Temperature Mutual Solubilities of Hydrocarbons and Water,” AIChE J. 29, 990–999 (1983).
S. Sourirajan and G. C. Kennedy, “The System H2O + NaCl at Elevated Temperatures and Pressures, Amer. J. Sci. 260, 115–141 (1960).
M. Yu. Belyakov S. B. Kiselev, and J. C. Rainwater, “Crossover Leung-Griffiths Model and Phase Behavior of Dilute Aqueous Ionic Solutions,” J. Chem. Phys. 107, 3085 (1997).
A. A. Povodyrev, M. A. Anisimov, J. V. Sengers, et al., Critical Locus of Aqueous Solutions of Sodium Chloride, Technical Report Prepared to IAPWS, IPST (Univ. of Maryland at College Park, 1998).
Y. Shibue, “Modified Rackett Equation Applied to Water and aqueous NaCl and KCl Solutions,” J. Chem. Eng. Data 45, 523–529 (2000).
K. S. Pitzer and R. T. Pabalan, “Thermodynamics of NaCl in Steam,” Geochim. et Cosmochim. Acta 50, 1445–1454 (1986).
C. S. Oakes, R. J. Bodnar, J. M. Simonson, and K. S. Pitzer, “Critical and Supercritical Properties for 0.3 to 3.0 mol kg−1 CaCl2 (aq),” Geochim. et Cosmochim. Acta 58, 2421–2431 (1994).
K. I. Shmulovich, S. I. Tkachenko, and N. V. Plyasunova, “Phase Equilibria in Fluid Systems at High Pressures and Temperatures,” in Fluids in the Crust: Equilibrium and Transport Properties, Ed. by K. I. Schmulovich et al. (Chapman & Hall, London, 1995), pp. 193–214.
Author information
Authors and Affiliations
Additional information
Original Russian Text © A.I. Abdulagatov, G.V. Stepanov, I.M. Abdulagatov, 2008, published in Teploenergetika.
Rights and permissions
About this article
Cite this article
Abdulagatov, A.I., Stepanov, G.V. & Abdulagatov, I.M. Critical properties of aqueous solutions. P. II. Therm. Eng. 55, 795–803 (2008). https://doi.org/10.1134/S0040601508090127
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040601508090127