Abstract
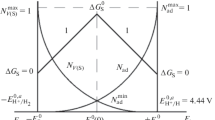
The adequacy of a stochastic model for choosing ligands which makes it possible to determine the regions of allowable values of the stability constants of complexes, the reduction of which occurs with the formation of an alloy, and choose ligands on the basis of these data is verified. Experimental data for alloy electrodeposition from solutions with complexes of different ligands and complexes of multivalent ions that are in published data show a good agreement with predictions by this model, which made it possible to formulate an important-for-practice condition of choosing ligands for joint reduction of complexes and the formation of an alloy.
Similar content being viewed by others
References
Vinokurov, E.G. and Bondar’, V.V., Logistic Model for Choosing Ligands for Alloy Electrodeposition, Teor. Osn. Khim. Tekhnol., 2007, vol. 41, no. 4, p. 384 [Theor. Found. Chem. Eng. (Engl. Transl.), vol. 41, no. 4, p. 384].
Diagrammy sostoyaniya dvoinykh metallicheskikh sistem. Spravochnik (Phase Diagrams of Double Metal Systems), Lyakishev, N.P., Ed., Moscow: Mashinostroenie, 1996.
Polukarov, Yu.M. and Gorbunova, K.M., Some Problems of the Theory of Alloy Electrodeposition, Zh. Fiz. Khim., 1956, vol. 30, no. 4, p. 878.
Spravochnik po elektrokhimii (Electrochemistry Handbook), Sukhotin, A.M., Ed., Leningrad: Khimiya, 1981.
Solov’eva, Z.A., Production of Iron-Gold Alloys by the Electrolytic Method, Zashchitnye metallicheskie i oksidnye pokrytiya, korroziya metallov i issledovaniya v oblasti elektrokhimii (Protective Metal and Oxide Coatings, Metal Corrosion, and Studies in the Field of Electrochemistry), Fedot’ev, N.P., Ed., Moscow: Nauka, 1965, p. 186.
Kudryavtsev, N.T., Kushevich, I.F., and Zhandarova, N.A., Electrolytic Deposition of the Silver-Palladium Alloy in the Ammine-EDTA Electrolyte, Zashch. Met., 1971, vol. 7, no. 2, p. 206.
Vinogradov, S.N. and Starikov, V.N., Electrodeposition of the Palladium-Copper Alloy from the Ammine-EDTA Electrolyte, Gal’vanotekh. Obrab. Poverkhn., 1997, vol. 5, no. 3, p. 22.
Vinogradov, S.N., Elektroosazhdenie splavov palladiya (Electrodeposition of Palladium Alloys), Polukarov, Yu.M., Ed., Saratov: Saratov. Univ., 1978.
Katarigi Iosio. Japanese Patent 25607, 1971.
Burkat, G.K., Physicomechanical Properties of the Palladium-Indium Alloy Obtained from the Ammine-Sulfosalicylate Electrolyte, Gal’vanotekh. Obrab. Poverkhn., 1994, vol. 3, nos. 5–6, p. 63.
Vinogradov, S.N. and Shumilina, N.I., Electrodeposition of the Palladium-Cadmium Alloy from the Ammine-EDTA Electrolyte, Zashch. Met., 1976, vol. 12, no. 4, p. 482.
Vinogradov, S.N., Kulagina, N.F., Ruzanova, G.G., and Kudryavtsev, N.T., Zashch. Met., 1972, vol. 8, no. 5, p. 611.
Susumu Arai, Hideki Akatsuka, Norio Kaneko, Sn-Ag Solder Bamp Formation for Flip-Chip Bonding by Electrodeposition, J. Electrochem. Soc., 2003, vol. 150, no. 10, p. C730.
Pronin, D.M., Rusanov, A.M., and Simoshina, A.G., in Sovremennye zashchitno-dekorativnye pokrytiya metallov (Modern Protective-Decorative Coatings of Metals), Moscow: MDNTP, 1974, p. 105.
Stabrovskii, A.I., Electrolytic Brass-Plating from Alkaline Cyanide-Free Solution, Zh. Prikl. Khim., 1952, vol. 25, no. 9, p. 966.
Garanina, I.I. and Gudin, N.V., Brass-Plating from the Glycinate-Ethylenediamine Electrolyte, Zashch. Met., 1985, vol. 21, no. 6, p. 931.
Boiko, I.A., Galinker, V.S., Duzhak, Yu.V., Kudra, O.K., and Matsola, M.V., Study of the Conditions of Electrodeposition of the Copper-Manganese Alloy from the EDTA Electrolyte, Zashch. Met., 1975, vol. 11, no. 1, p. 103.
Graham, A.K., Heiman, S.U., and Pinkerton, H.L., Deposition of Precious Metal Alloys. II. Binary Silver Alloys from Acid Chloride Solution. III. Pt-Au, Ag-Au and Ag-Pt-Au Alloys from Acid Chloride Solution, Plating, 1949, vol. 36, no. 1, p. 47; no. 2, p. 148.
Parker, E.A., Recent Developments in Gold-Alloy Plating, Plating, 1958, vol. 45, no. 6, p. 631.
Khotyanovich, S.I., Elektroosazhdenie metallov platinovoi gruppy (Electrodeposition of Platinum-Group Metals), Vilnius: Mokslas, 1976.
Andryushchenko, F.K., Orekhova, V.V., and Pavlovskaya, K.K., Pirofosfatnye elektrolity (Pyrophosphate Electrolytes), Kiev: Tekhnika, 1965.
Vinogradov, S.N., Suvorova, G.P., Vershinina, L.P., and Dedushek, N.F., Electrodeposition of the Palladium-Tin Alloy from the EDTA Electrolyte, Zashch. Met., 1976, vol. 12, no. 2, p. 207.
Vinogradov, S.N., Yakovleva, E.G., and Ogurtsova, V.L., Electrodeposition of the Palladium-Cobalt Alloy, in Teoriya i praktika elektroosazhdeniya metallov i splavov (Theory and Practice of Electrodeposition of Metals and Alloys), Penza: Privolzhskoe Knizjnoe Izd., Penzenskoe otd., 1976, p. 40.
Brenner, A., Electrodeposition of Alloys, New York: Academic, 1963, vol. 1.
Abd. El-Rehim S.S., Away A., and El-Sayd A., Electroplating of Indium and Indium-Tin Alloys from Alkaline Bath, Hung. J. Ind. Chem, 1988, vol. 16, no. 44, p. 419.
Perelygin, Yu.P., Elektroosazhdenie, svoistva i oblast’ primeneniya indiya i ego dvoinykh splavov (Properties and Application of Indium and Its Binary Alloys), Penza: Penz. Politekh. Inst, 1993.
Izmailov, A.V., Chernysheva, N.P., and Pronyuk, V.G., Corrosion Tests of the Chromium-Tin Alloy, Izv. Vyssh. Uchebn. Zaved. Pishch. Tekhnol., 1967, no. 6, p. 119.
Sillen, L.G. and Martel, A.E., Stability Constants of Metal-Ion Complexes, Spec. Publ. No. 17, London: Chemical Society, 1964.
Perrin, D.D., Stability Constants of Metal-Ion Complexes., Oxford: Pergamon, 1983, part B.
Lur’e, Yu.Yu., Spravochnik po analiticheskoi khimii (Analytical Chemistry Handbook), Moscow: Khimiya, 1989.
Pyatnitskii, I.V., Complex Compounds of Metals with Oxyacids, Usp. Khim., 1963, vol. 32, no. 1, p. 93.
Vinokurov, E.G. and Bondar’, V.V., Prediction of Stability Constants of the Complexes of Chromium(III) and Chromium( II), Koord. Khim., 2003, vol. 29, no. 1, p. 71.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E. G. Vinokurova and V. V. Bondar’, 2009, published in Teoreticheskie Osnovy Khimicheskoi Tekhnologii, 2009, Vol. 43, No. 3, pp. 308–312.
Rights and permissions
About this article
Cite this article
Vinokurov, E.G., Bondar’, V.V. Adequacy of a stochastic model for choosing ligands for alloy electrodeposition from solutions of mixed-ligand and multivalent ion complexes. Theor Found Chem Eng 43, 293–297 (2009). https://doi.org/10.1134/S0040579509030087
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0040579509030087