Abstract
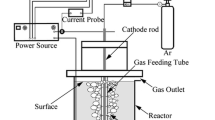
The chlorine utilization efficiency and singlet oxygen concentration are determined as functions of the chlorine flow rate, the base concentration, and the flow rate and temperature of basic hydrogen peroxide in a singlet oxygen generator based on the reaction of chlorine with filament-guided descending basic hydrogen peroxide jets. The effluent gas, without aerosol entrainment, contains about 10% residual chlorine and more than 60% singlet oxygen.
Similar content being viewed by others
References
Khan, A.U., Singlet Molecular Oxygen: A New Kind of Oxygen, J. Phys. Chem., 1976, vol. 80, no. 20, p. 2219.
McDermott, W.E., Pchelkin, N.R., Benard, D.J., and Bousek, R.R., An Electronic Transition Chemical Laser, Appl. Phys. Lett., 1978, vol. 32, no. 8, p. 469.
Browne, R.J. and Ogryzlo, E.A., Chemiluminescence from the Reaction of Chlorine with Aqueous Hydrogen Peroxide, Proc. Chem. Soc., 1964, p. 117.
Azyazov, V.N., Zagidullin, M.V., Nikolaev, V.D., Svistun, M.I., and Khvatov, N.A., Jet Generator ofO2(1Δ) with an Oxygen Pressure to 13.3 kPa, Kvantovaya Elektron., 1994, vol. 21, no. 2, p. 129.
Balej, J. and Spalek, O., Calculation of Equilibrium Composition in More Concentrated SystemsH2O2-KOH (or NaOH)-H2O, Collection Czechoslov. Chem. Commun., 1979, vol. 44, p. 488.
Richardson, R.J., Kelley, J.D., and Wiswall, C.E., O2(1Δ) Generation Mechanisms in the Chemically Pumped Iodine Laser, J. Appl. Phys., 1981, vol. 52, no. 2, p. 1066.
Storch, D.M., Dymek, C.J., and Davis, L.P., MNDO Study of the Mechanism of O2(1Δ) Formation by Reaction of Cl2 with Basic H2O2 // J. Am. Chem. Soc., 1983, vol. 105, p. 1765.
Azyazov, V.N., Zagidullin, M.V., Nikolaev, V.D., and Ufimtsev, N.I., Kinetics of Chemisorption of Cl2 by H2O-H2O2-KOH and H2O-KOH Solutions, Zh. Fiz. Khim., 1998, vol. 72, no. 10, p. 1850 [Russ. J. Phys. Chem. (Engl. Transl.), vol. 72, no. 10, p. 1681].
Gershenzon, M., Davidovits, P., Jayne, J.T., Kolb, C.E., and Worsnop, D.R., Rate Constant for the Reaction of Cl2(aq) with OH−, J. Phys. Chem. A, 2002, vol. 106, p. 7748.
Rogers, M.A., Lifetime ofO2(1Δ) in Liquid Water as Determined by Time Resolved Infrared Luminescence Measurements, J. Am. Chem. Soc., 1982, vol. 104, no. 20, p. 5541.
Basov, N.G., Zagidullin, M.V., Igoshin, V.I., Katulin, V.A., and Kupriyanov, N.L., Theoretical Analysis of Chemical Oxygen-Iodine Lasers, Tr. Fiz. Inst. im. P.N. Lebedeva, Akad. Nauk SSSR, 1986, vol. 171, p. 30.
Blauer, J.A., Munjee, S.A., Truesdell, K.A., Curtis, E.C., and Sullivan, J.F., Aerosol Generators for Singlet Oxygen Production, J. Appl. Phys., 1987, vol. 62, no. 6, p. 2508.
Harpole, G.M., English, W.D., Berd, J.G., and Miller, D.J., Rotating Disk Oxygen Generator, AIAA Paper 92-3006, in 23rd Plasmadynamics and Lasers Conference, Nashville, Tenn., United States, July 6–8, 1992.
Vetrovec, J., Singlet Oxygen Generator with Filament-Guided Jets, Proc. SPIE, 1998, vol. 3574, p. 546.
Okabe, H., Photochemistry of Small Molecules, New York: Wiley, 1978. Translated under the title Fotokhimiya malykh molekul, Moscow: Mir, 1981.
Yang, T.T., Copeland, D.A., Bauer, A.H., Quan, V., McDermott, W.E., Cover, R.A., and Smith, D.M., Chemical Oxygen-Iodine Laser Performance Modeling, AIAA Paper 97-2384, in 28th Plasmadynamics and Lasers Conference, Atlanta, Ga., United States, June 23–25, 1997.
Dankwerts, P.V., Gas-Liquid Reactions, New York: McGraw-Hill, 1970. Translated under the title Gazozhidkostnye reaktsii, Moscow: Khimiya, 1973.
Hager, G.D., Nikolaev, V.D., Svistun, M.I., and Zagidullin, M.V., Lasing Performance of a Chemical Oxygen Iodine Laser (COIL) with Advanced Ejector Nozzle Banks, Appl. Phys. A, 2003, vol. 77, p. 325.
Author information
Authors and Affiliations
Additional information
Original Russian Text © M.V. Zagidullin, V.D. Nikolaev, M.I. Svistun, N.A. Khvatov, 2007, published in Teoreticheskie Osnovy Khimicheskoi Tekhnologii, 2007, Vol. 41, No. 2, pp. 176–182.
Rights and permissions
About this article
Cite this article
Zagidullin, M.V., Nikolaev, V.D., Svistun, M.I. et al. Singlet oxygen generator based on the reaction of chlorine with filament-guided jets of basic hydrogen peroxide. Theor Found Chem Eng 41, 164–170 (2007). https://doi.org/10.1134/S0040579507020091
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1134/S0040579507020091