Abstract
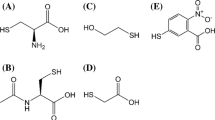
This present study aims to report the spectral, kinetic, and electrochemical results of the selective oxidation of substrates such as L-methionine (I), L-ethinonine (II), L-buthionine (III), and N-acetyl-L-methionine (IV) with [O=FeIV-salen]\(^{{ \bullet + }}\) oxidant species 1a–5a in aqueous CH3CN under biological conditions. Reactions between oxidants and the substrates follow saturation kinetics (Michalis-Menton type), and this reaction is very sensitive with the substituents present in salen ligand of the complex, structure of substrates I–IV, the effects of pH, substrate concentration, the polarity of the solvent, and temperature. The [O=FeIV-salen]\(^{{ \bullet + }}\) oxidant species is confirmed by spectral and electrochemical techniques. The positive reaction constants (ρ = 0.79–0.90) are obtained from the plot of rate constant (k) values versus substituent constants (σ) values, which indicates the development of negative charge in the transition state (TS). The formation of the product during the course of reaction is analyzed by FT-IR, ESI-MS, and 1H-NMR techniques. Based on the experimental evidences a plausible electron transfer (ET) and oxygen atom transfer (OAT) mechanisms have been proposed.

















Similar content being viewed by others
REFERENCES
X. Lu, Y. Lee, M. S. Seo, and W. Nam, Chem. Commun. 76, 11207 (2020). https://doi.org/10.1039/D0CC05145D
T. R. Ward, ACS Cent. Sci. 5, 1732 (2019)
L. Vicens, G. Olivo, and M. Costas, ACS Catal. 10, 8611–8631 (2020). https://doi.org/10.1021/ACSCatal.0c02073
V. A. Larson, B. Battistella, K. Ray, et al., Nat. Rev. Chem. 4, 404 (2020).
G. Olivo, O. Cussó, M. Borrell, and M. Costas, J. Biol. Inorg. Chem., Nos. 2–3, 425 (2017).
S. P. de Visser and D. Kumar, Iron-Containing Enzymes Versatile Catalysts of Hydroxylation Reaction in Nature (RSC, Cambrige, UK, 2011).
J. C. Lewis, ACS Catal. 3, 2954 (2013).
S. Shaik, S. Cohen, Y. Wang, et al., Chem. Rev. 110, 949 (2010).
V. K. Sivasubramanian, M. Ganesan, S. Rajagopal, and R. Ramaraj, J. Org. Chem. 67, 1506 (2002).
A. Mary Imelda Jayaseeli and S. Rajagopal, J. Mol. Catal. A: Chem. 309, 103 (2009).
A. Mohamed Aslam, S. Rajagopal, M. Vairamani, and M. Ravikumar, Trans. Met. Chem. 36, 751 (2011).
R. M. Clarke, K. Herasymchuk, and T. Storr, Coord. Chem. Rev. 352, 67 (2017).
A. Chellamani, N. M. I. Alhaji, and S. Rajagopal, J. Chem. Soc. Perkin Trans. 2, 299 (1997).
J. C. Pessoa and I. Correia, Coord. Chem. Rev. 388, 227 (2019).
N. S. Venkataramanan, S. Premsingh, S. Rajagopal, and K. Pitchumani, J. Org. Chem. 68, 7460 (2003).
A. Erxleben, Inorg. Chim. Acta 472, 40 (2018).
A. Lidskog, Y. Li, and K. Wärnmark, Catalysts 10, 705 (2020).
N. S. Venkataramanan, S. Rajagopal, and M. Vairamani, J. Inorg. Biochem. 101, 274 (2007).
A. Chellamani, N. Ismail Alhaji, and S. Rajagopal, J. Phys. Org. Chem. 20, 255 (2007).
D. Thiruppathi, P. Karuppasamy, M. Ganesan, et al., Int. J. Chem. Kinet. 46, 606 (2014).
D. Thiruppathi, P. Karuppasamy, M. Ganesan, et al., J. Photochem. Photobiol. A 295, 70 (2014).
C. Kavitha and P. Subramaniam, Polyhedron 189, 114712 (2020).
C. Kavitha and P. Subramaniam, Polyhedron 175, 114172 (2020).
G. Olivo, O. Lanzalunga, and S. D. Stefano, Adv. Synth. Catal. 358, 843 (2016).
H. Pellissier, Coord. Chem. Rev. 284, 93 (2015).
E. I. Solomon, Inorg. Chem. 40, 3656 (2001).
M. J. Ryle and R. P. Hausinger, Curr. Opin. Chem. Biol. 6, 193 (2002).
C. A. Grapperhaus, B. Mienert, E. Bill, et al., Inorg. Chem. 39, 5306 (2000).
Y. M. Kim, K. B. Cho, J. Cho, et al., J. Am. Chem. Soc. 135, 8838 (2013).
S. P. de Visser, J. U. Rohde, Y. M. Lee, et al., Coord. Chem. Rev. 257, 381 (2013).
A. R. McDonald and L. Que, Jr., Coord. Chem. Rev. 257, 414 (2013).
S. Shaik, S. Cohen, Y. Wang, et al., Chem. Rev. 110, 949 (2010).
A. Gunay and K. H. Theopold, Chem. Rev. 110, 1060 (2010).
C.-M. Che, V. K. Y. Lo, C. Y. Zhou, and J. S. Huang, Chem. Soc. Rev. 40, 1950 (2011).
M. Costas, Coord. Chem. Rev. 255, 2912 (2011).
K. Cho, P. Leeladee, A. J. McGown, et al., J. Am. Chem. Soc. 134, 7392 (2012).
S. Hong, Y. M. Lee, K. B. Cho, et al., J. Am. Chem. Soc. 133, 11876 (2011).
H. Tang, J. Guan, L. Zhang, et al., Phys. Chem. Chem. Phys. 14, 12863 (2012).
D. Kumar, G. N. Sastry, and S. P. de Visser, J. Phys. Chem. B 116, 718 (2012).
T. Ohta, J. G. Liu, and Y. Naruta, Coord. Chem. Rev. 257, 407 (2013).
W. Nam, Y. M. Lee, and S. Fukuzumi, Acc. Chem. Res. 47, 1146 (2014).
E. R. Stadtman, H. V. Remmen, A. Richardson, et al., Biochim. Biophys. Acta 1703, 135 (2005).
B. C. Lee, A. Dikiy, H. Y. Kim, and V. N. Gladyshev, Biochim. Biophys. Acta 1790, 1471 (2009).
H. Y. Kim and V. N. Gladyshev, Mol. Biol. Cell 15, 1055 (2004).
J. D. Meyer, B. Ho, and M. C. Manning, Rational Design of Stable Protein Formulations: Theory and Practice, Ed. by J. F. Carpenter and M. C. Manning (Kluwer Academic, Plenum, New York, 2002), p. 85.
D. A. Ferrington, H. Sun, K. K. Murray, et al., J. Biol. Chem. 276, 937 (2001).
N. R. Matheson and P. S. Wong, J. Biochem. Biophys. Res. Commun. 88, 402 (1979).
L. C. The, L. J. Murphy, N. L. Huq, et al., J. Biol. Chem. 262, 6477 (1987).
R. A. Depaz, C. C. Barnett, D. A. Dale, et al., Arch. Biochem. Biophys. 384, 123 (2000).
H. S. Lu, P. R. Fausset, L. O. Narthi, et al., Arch. Biochem. Biophys. 362, 1 (1999).
M. J. Wood, J. Helena Prieto, and E. A. Komives, Biochem. Biophys. Acta 1703, 141 (2005).
E. R. Stadtman and C. N. Oliver, J. Biol. Chem. 266, 2005 (1991).
E. R. Stadtman, Science (Washington, DC, U. S.) 257, 1220 (1992).
K. Merker, N. Sitte, and T. Grune, Arch. Biochem. Biophys. 375, 50 (2000).
M. J. Hokenson, V. N. Uversky, J. Goers, et al., Biochemistry 43, 4621 (2004).
W. R. Markesbery, Free Radical. Biol. Med. 23, 134 (1997).
D. A. Butterfield and R. Sultana, J. Amino Acids 2011, 10 (2011).
J. Hong and C. Schöneich, Free Radical. Biol. Med. 31, 1432 (2001).
A. I. Abouelatta, A. A. Campanali, A. R. Ekkati, et al., Inorg. Chem. 48, 7729 (2009).
P. Karuppasamy, D. Thiruppathi, J. Vijaya Sundar, et al., Arab. J. Sci. Eng. 40, 2945 (2015).
N. Hessenauer-Ilicheva, A. Franke, D. Meyer, et al., J. Am. Chem. Soc. 129, 12473 (2007).
P. Karuppasamy, D. Thiruppathi, J. Vijaya Sundar, et al., Polyhedron 114952, 196 (2021),
P. Karuppasamy, D. Thiruppathi, M. Ganesan, et al., Polyhedron 159, 135 (2019).
S. W. Griffiths and C. L. Cooney, Biochemistry 41, 6245 (2002).
E. Derat, S. Shaik, C. Rovira, et al., J. Am. Chem. Soc. 129, 6346 (2007).
T. Kurahashi, Y. Kobayashi, S. Nagamoto, et al., Inorg. Chem. 44, 8156 (2005).
T. A. Enache and A. M. Oliveira-Brett, Bioelectrochem. 81, 46 (2011).
J. B. Raoof, R. Ojani, and Z. Mohammadpour, Anal. Bioanal. Electrochem. 2, 24 (2010).
H. Sugimoto, H. Tung, and D. H. Sawyer, J. Am. Chem. Soc. 110, 2465 (1988).
A. Takahashi, T. Kurahashi, and H. Fujii, Inorg. Chem. 50, 6922 (2011).
T. M. Goto, S. Ozaki, Y. Watanabe, and S. Fukuzumi, J. Am. Chem. Soc. 121, 9497 (1999).
S. W. Griffiths and C. L. Cooney, Biochemistry 41, 6245 (2002).
E. Derat, S. Shaik, C. Rovira, et al., J. Am. Chem. Soc. 129, 6346 (2007).
R. Zhang, J. H. Horner, and M. Newcomb, J. Am. Chem. Soc. 127, 6573 (2005).
T. Kurahashi, Y. Kobayashi, S. Nagamoto, et al., Inorg. Chem. 44, 8156 (2005).
D. Selmar, in Annual Plant Reviews, Vol. 40: Biochemistry of Plant Secondary Metabolism, Ed. by O. Wink, 2nd ed. (Wiley, Chichester, 2010), Chap. 3, p. 92.
ACKNOWLEDGMENTS
The author thanks the PG and Research Department of Chemistry, Vivekananda College, Tiruvedakam West, Madurai-625 234 for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Rights and permissions
About this article
Cite this article
Karuppasamy, P. Selective Oxidation of L-Methionine, L-Ethionine, N-Acetyl-L-Methionine, L-Buthionine Catalyzed by [FeIII-Salen]Cl Complexes: A Spectral, Kinetic, and Electrochemical Study. Russ. J. Phys. Chem. 95 (Suppl 2), S230–S241 (2021). https://doi.org/10.1134/S0036024421150127
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421150127