Abstract
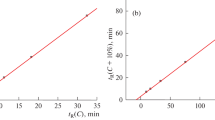
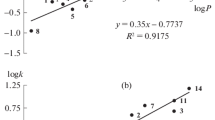
Recurrent approximation of retention times in reversed-phase high performance liquid chromatography (RP-HPLC), tR(C + ΔC) = atR(C) + b, where C is the acetonitrile concentration in the eluent, and ΔC is the constant “step” of its variation, for six specially synthesized N-substituted p-toluenesulfonamides confirmed the presence of anomalies previously revealed for some complex polyfunctional organic compounds. The reason for these anomalies is the presence of sulfonamide –SO2–NH fragments in the molecules, which leads to hydration of sorbates in aqueous solutions, or, more precisely, to a change in the ratio of their non-hydrated and hydrated forms because of a shift in the equilibrium Х + Н2О \( \rightleftarrows \) Х·Н2О (*) as a result of a change in the eluent composition. The same effect is indicated by the strong antibatic dependence of the retention indices RI(C) of all sulfonamides under study; the coefficients dRI/dC vary from –1.9 to –4.0, these values being much higher in magnitude than for compounds that do not form hydrates. Further independent evidence in favor of the transformation of sorbates due to variation of the eluent composition is the dependence of the relative absorbance Arel = A(254)/A(220) on the acetonitrile content in the eluent. This suggests changes in the chemical nature of chromophores in sulfonamide molecules depending on the equilibrium state (*).









Similar content being viewed by others
Notes
For retention indices, IUPAC recommends the one-letter symbol “I.” However, it has other meanings (electric current, radiation intensity, peak intensity in mass spectra, etc.); as a result, expressions containing “I” symbols with different meanings can appear in chromatography. Therefore, we prefer the two-letter symbol RI.
REFERENCES
J. D. Rawn and R. Quellette, Organic Chemistry, 2nd ed. (Academic, New York, 2019).
I. G. Zenkevich and D. A. Nikitina, Russ. J. Phys. Chem. A 95, 395 (2021). https://doi.org/10.1134/S003602442102028X
M.-L. Guo, Crystallogr. Commun. 60, 574 (2004). https://doi.org/10.1107/S1600536804005446
S. F. Suchetan, S. Foro, B. T. Gowda, and M. S. Prakash, Acta Crystallogr., Sect. E 68, o46 (2012). https://doi.org/10.1107/S1600536811051932
A. Kompella, S. Kasa, V. S. Balina, et al., Sci. J. Chem. 2 (6–1), 9 (2014). https://doi.org/10.11648/j.sjc.s.2014020601.12
M. Jatezak, K. Sidoryk, M. Kossykowska, et al., Chromatographia 79, 1131 (2016). https://doi.org/10.1007/s10337-016-3124y
E. Jurczak, A. H. Mazurek, L. Szeleszczuk, et al., Pharmaceuticals 12, 1 (2020). https://doi.org/10.3390/pharmaceuticals12100959
I. G. Zenkevich, J. Chemometr. 23, 179 (2009). https://doi.org/10.1002/cem.1214
I. G. Zenkevich, in Chemometrics in Chromatography, Ed. by L. Komsta, Y. V. Heyden, and J. Sherma (CRC, London, 2018), Chap. 24, p. 449.
I. G. Zenkevich, J. Chemometr. 24, 158 (2010). https://doi.org/10.1002/cem.1297
T. A. Kornilova, A. Deruish, and I. G. Zenkevich, Anal. Kontrol’ 24, 315 (2020). https://doi.org/10.15826/analitika.2020.24.4.006
M. L. Peterson and J. Hirsch, J. Lipid Res. 1, 132 (1959).
Yu. V. Patrushev, Yu. S. Sotnikova, and V. N. Sidel’nikov, Prot. Met. Phys. Chem. Surf. 56, 49 (2020). https://doi.org/10.1134/S2070205119060248
S. N. Lanin, M. Y. Ledenkova, and Y. S. Nikitin, J. Chromatogr. A 797, 3 (1998).
W. Zapala, J. Chromatogr. Sci. 41, 289 (2003).
V. A. Chirkin, S. I. Karpov, and V. F. Selemenev, J. Anal. Chem. 68, 341 (2013). https://doi.org/10.1134/S10619348113020056
B. R. Saifutdinov, V. A. Davankov, and M. M. Il’in, Russ. J. Phys. Chem. A 88, 358 (2014). https://doi.org/10.1134/S0036024414030224
B. R. Saifutdinov, V. A. Davankov, G. A. Petukhova, M. P. Tsyurupa, Z. K. Blinnikova, and M. M. Il’in, Dokl. Phys. Chem. 462, 135 (2015). https://doi.org/10.1134/S0012501615060056
A. S. Savchenkova, A. K. Buryak, and S. V. Kurbatova, Russ. J. Phys. Chem. A 89, 1662 (2015). https://doi.org/10.1134/S0036024415090277
I. Canals, J. A. Portal, M. Roses, and E. Bosch, Chromatographia 55, 565 (2022).
N. Sanli, S. Sanli, G. Ozkan, and A. Denizli, J. Braz. Chem. Soc. 21, 1952 (2010).
M. Remko, J. Mol. Struct.: THEOCHEM 941, 34 (2010). https://doi.org/10.1016/j.theochem.2009.12.017
B. A. Caine, M. B. Bronzato, and L. A. Popelier, J. Chem. Sci. 10, 6368 (2019). https://doi.org/10.1039/c9sc01818b
I. G. Zenkevich, M. V. Kochetova, O. G. Larionov, et al., J. Liq. Chromatogr. Rel. Technol. 28, 2141 (2005). https://doi.org/10.1081/JLC-200064000
Encyclopedia of Chromatography, Ed. by J. Cazes, 3rd ed. (Taylor and Francis, New York, 2010), Vol. 2, p. 1304.
Retention and Selectivity in Liquid Chromatography, Vol. 57 of J. Chromatogr. Libr., Ed. by R. M. Smith (Elsevier, Amsterdam, 1995), p. 93.
Handbuch der Gaschromatographie, Herausgegeben, Ed. by E. Leibnitz and H. G. Struppe (Akademische Verlag, Leipzig, 1984).
I. G. Zenkevich and V. M. Kosman, Russ. J. Appl. Chem. 70, 1770 (1997).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Smolina
Rights and permissions
About this article
Cite this article
Zenkevich, I.G., Nikitina, D.A. & Kornilova, T.A. Recurrent Approximation of Retention Parameters of N-Substituted p-Toluenesulfonamides in Reversed-Phase High Performance Liquid Chromatography for Revealing the Formation of Their Hydrates. Russ. J. Phys. Chem. 95, 1931–1941 (2021). https://doi.org/10.1134/S0036024421090326
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421090326