Abstract
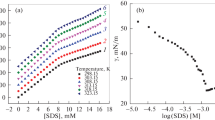
The densities of solutions of L-tryptophan (Trp) in water and sodium dodecyl sulfate (SDS) in Trp aqueous solutions are measured in a wide range of temperatures (293.15, 298.15, 303.15, 308.15, and 313.15) K using a DMA 5000 М density meter (Anton Paar).The apparent molar volumes (\({{V}_{{\varphi ,{\text{SDS}}}}}\)) of sodium dodecyl sulfate in the 0.0010–0.0199 mol kg−1 range of concentrations and its limiting apparent molar volumes at infinite dilution (\(V_{{\varphi ,{\text{SDS}}}}^{^\circ }\)) in solutions containing the amino acid (at a fixed concentration of 0.01 mol kg–1) are determined. A rise in the first critical micelle concentration from m = 0.0080 for SDS solutions in water to m = 0.0099 for SDS solutions with Trp is observed. Values of derivatives (\(\partial V_{{\varphi ,{\text{SDS}}}}^{^\circ }\)/∂T)p and (\({{\partial }^{2}}V_{{\varphi ,{\text{SDS}}}}^{^\circ }\)/∂T 2)p, and the partial molar volumes of SDS transfer from water to amino acid aqueous solutions, are calculated. Results are discussed by considering different types of intermolecular interaction in the given solutions. Quantum-chemical DFT/B97D modeling of the complexes between SDS and L-tryptophan zwitterion is done using a combination of basis set 6-311++G(2d,2p) and Grimme’s functional hybrid exchange correlation with a dispersion correction. The polarizable continuum model (PCM) is used to determine the structure and energies of formation of the SDS…Trp complexes with allowance for hydration effects.




Similar content being viewed by others
REFERENCES
Antimicrobial Peptides: Methods and Protocols, Ed. by A. Giuliani and A. C. Rinaldi (Humana, New York, 2010).
M. R. Bozorgmehr, M. Saberi, and H. Chegini, J. Mol. Liq. 199, 184 (2014).
Y. Ding, Y. Shu, L. Ge, and R. Guo, Colloids Surf., A 298, 163 (2007).
Z. Liu, X. Guo, Z. Feng, and L. Jia, J. Solution Chem. 44, 293 (2015).
H. D. Thaker, F. Sgolastra, D. Clements, R. W. Scott, and G. N. Tew, J. Med. Chem. 54, 2241 (2011).
I. M. Yermak and V. N. Davydova, Biochemistry (Moscow) Suppl. Ser. A: Membr. Cell Biol. 2, 279 (2008).
E. G. Sumina, S. N. Shtykov, and N. V. Tyurina, J. Anal. Chem. 58, 720 (2003).
J. L. Brash and T. A. Horbett, Proteins at Interfaces II: Fundamentals and Applications (Am. Chem. Soc., Washington DC, 1995).
E. G. Sumina, in Nanoanalytics, Nanoobjects, and Nanotechnologies in Analytical Chemistry, Ed. by S. Shtykov (De Gruyter, Berlin, 2018), p. 411.
M. G. Khaledi and A. H. Rodgers, Anal. Chim. Acta 239, 121 (1990).
R. Badarayani and A. Kumar, J. Chem. Thermodyn. 36, 49 (2004).
O. S. Chernysheva and Ya. A. Maslova, Colloid. J. 78, 407 (2016).
E. G. Sumina, O. N. Sorokina, V. Z. Uglanova, and T. E. Sorokina, Ital. Sci. Rev. 26 (5), 101 (2015).
S. K. Singh, A. Kundu, and N. Kishore, J. Chem. Thermodyn. 36, 7 (2004).
S. Chauhan, M. S. Chauhan, P. Sharma, D. S. Rana, and A. Umar, Fluid Phase Equilib. 337, 39 (2013).
Z. Yan, X. Sun, W.-W. Li, Y. Li, and J. Wang, J. Chem. Thermodyn. 13, 1468 (2011).
A. Ali, N. A. Malik, S. Uzair, and M. Ali, Mol. Phys. 112, 2681 (2014).
M. S. Hossain, T. K. Biswas, D. Ch. Kabiraz, Md. N. Islam, and M. E. Huque, J. Chem. Thermodyn. 71, 6 (2014).
M. Bello, G. Gutierres, and E. Garcia-Hernandez, Biophys. Chem. 165–166, 79 (2012).
P. Talele and N. Kishore, J. Chem. Thermodyn. 70, 182 (2014).
V. G. Zavodinskii, A. A. Gnidenko, and V. N. Davydova, Butler. Soobshch., No. 2, 11 (2003).
D. E. Nolde, P. E. Volynskii, A. S. Arsen’ev, and R. G. Efremov, Russ. J. Bioorg. Chem. 26, 115 (2000).
A. S. Khamidullina, I. V. Vakulin, R. F. Talipov, and I. S. Shepelevich, J. Struct. Chem. 46, 985 (2005).
Z. Qiu, Y. Xia, H. Wang, and K. Diao, J. Struct. Chem. 52, 462 (2011).
E. N. Brodskaya, Colloid. J. 74, 154 (2012).
V. G. Badelin, E. Yu. Tyunina, and G. N. Tarasova, Zh. Fiz. Khim. 91, 862 (2017).
N. I. Giricheva, M. S. Kurbatova, E. Yu. Tyunina, and V. G. Badelin, J. Struct. Chem. 58, 1604 (2017).
A. D. Zimon, A. M. Evtushenko, and I. G. Krasheninnikova, Physical and Colloid Chemistry, Practical Guide (MGUTU, Moscow, 2004) [in Russian].
X. Tang, P. H. Koenig, and R. G. Larson, J. Phys. Chem. B 118, 3864 (2014).
Yu. A. Ershov, Colloid Chemistry. Physical Chemistry of Dispersed Systems (GEOTAR-Media, Moscow, 2013) [in Russian].
K. Holmberg, B. Jonsson, B. Kronberg, and B. Lindman, Surfactants and Polymers in Aqueous Solution (Wiley, Chichester, UK, 1998).
H. Nagai, K. Kuwabara, and G. Carta, J. Chem. Eng. Data 53, 619 (2008).
Chemistry and Biochemistry of the Amino Acids, Ed. by G. C. Barrett (Chapman and Hall, London, New York, 1985).
V. P. Vasil’ev, V. A. Borodin, and E. V. Kozlovskii, Computers in Chemicoanalytical Calculations (Vysshaya Shkola, Moscow, 1993) [in Russian].
G. S. Kell, J. Chem. Eng. Data 20, 97 (1975).
R. Ditchfield, W. J. Hehre, and J. A. Pople, J. Chem. Phys. 54, 724 (1971).
M. J. Frisch, G. W. Truck, H. B. Schlegel, et al., Gaussian 09, Rev. D.01 (Gaussian Inc., Wallingford CT, 2013).
J. Tomasi, B. Mennucci, and R. Cammi, Chem. Rev. 105, 2999 (2005).
H. S. Harned and B. B. Owen, The Physical Chemistry of Electrolytic Solutions (Reinhold, New York, 1950).
F. J. Millero, Chem. Rev. 71, 147 (1971).
H. Zhao, Biophys. Chem. 122, 157 (2006).
E. Forgács, Fresenius J. Anal. Chem. 349, 743 (1994).
Theoretical and Experimental Methods of Chemistry of Solutions (Problems of Chemistry of Solutions), Ed. by A. Yu. Tsivadze (Prospekt, Moscow, 2011) [in Russian].
D. O. Masson, Philos. Mag. 8, 218 (1929).
L. Lepori and P. Gianni, J. Solution Chem. 29, 405 (2000).
F. Franks, Water: A Comprehensive Treatise (Plenum, New York, 1973), Vol. 3.
R. W. Gurney, Ionic Processes in Solution (McGraw-Hill, New York, 1953).
S. K. Singh, A. Kundu, and N. Kishore, J. Chem. Thermodyn. 36, 7 (2004).
Z. Yan, Q. Zhang, W.-W. Li, and J. Wang, J. Chem. Eng. Data 55, 3560 (2010).
N. G. Harutyunyan, L. R. Harutyunyan, and R. S. Harutyunyan, Thermochim. Acta 498, 124 (2010).
L. G. Hepler, Can. J. Chem. 47, 4613 (1969).
N. V. Usol’tseva, A. I. Smirnova, N. V. Zharnikova, et al., Zhidk. Krist. Prakt. Ispol’z. 16, 70 (2016).
The Cambridge Crystallographic Data Centre, Leibniz Institute for Information Infrastructure. www.ccdc.cam.ac.uk/structures/searchıd=doi:https://doi.org/10.5517/cc123lsk&sid=DataCite.
V. G. Badelin, E. Yu. Tyunina, I. N. Mezhevoi, and G. N. Tarasova, Russ. J. Phys. Chem. A 89, 2229 (2015).
V. V. Dunaeva, G. V. Girichev, and N. I. Giricheva, Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 63 (3), 37 (2020).
ACKNOWLEDGMENTS
Our densimetry studies were performed on equipment at the shared resource center of the Krestov Institute of Solution Chemistry, Upper Volga Region Center of Physicochemical Research (http://www.isc-ras.ru/ru/struktura/ckp).
Funding
The work was supported by the Russian Foundation for Basic Research, grant no. 18-03-01032.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by Z. Smirnova
Rights and permissions
About this article
Cite this article
Kurbatova, M.S., Tarasova, G.N., Tyunina, E.Y. et al. Investigation of Interactions between Sodium Dodecyl Sulfate and L-Tryptophan Through Densimetry and Computer Modeling. Russ. J. Phys. Chem. 95, 1606–1613 (2021). https://doi.org/10.1134/S0036024421080161
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421080161