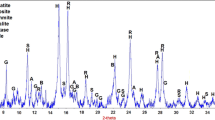
Abstract
The kinetics are studied of topochemical reactions of the formation of calcium hydrosilicate in a multicomponent aqueous system consisting of waste products of boric acid production. The waste products include silicon dioxide and calcium sulfate in equal molar fractions, an alkali metal hydroxide in a stoichiometric ratio to the molar content of calcium sulfate and silicon oxide for obtaining hydrosilicate calcium, and the volume of water calculated to obtain a solution of alkali metal sulfate with a concentration below saturation. Data are obtained on the kinetics of the formation of calcium hydrosilicates in different modes of processing (normal conditions with constant stirring; ultrasonic action at a temperature of 20°C; microwave exposure with constant stirring at a temperature of 95°C; and autoclaving at a temperature of 220°C). It is found that the fastest reaction rate is characteristic of microwave treatment, and the largest proportion of reacted alkali metal hydroxide is obtained during autoclave treatment.




Similar content being viewed by others
REFERENCES
P. S. Gordienko, I. A. Shabalin, S. B. Yarusova, I. G. Zhevtun, and O. S. Vasilenko, Russ. J. Phys. Chem. A 93, 2284 (2019). https://doi.org/10.1134/S0036024419110116
S. B. Yarusova, N. V. Makarenko, P. S. Gordienko, M. A. Karpenko, and E. S. Novikova, Russ. J. Phys. Chem. A 92, 559 (2018). https://doi.org/10.1134/S0036024418030354
P. S. Gordienko, S. B. Yarusova, A. P. Suponina, et al., Theor. Found. Chem. Eng. 49, 726 (2015). https://doi.org/10.1134/S0040579515050061
R. M. Alosmanov, Sorbtsion. Khromatogr. Prots. 10, 427 (2010).
M. Bouatrous, F. Bouzerara, A. K. Bhakta, et al., Ceram. Int. 46 (Part B), 12618 (2020). https://doi.org/10.1016/j.ceramint.2020.02.026
J. Ma, G. Qin, Y. Zhang, et al., J. Clean. Prod. 182, 776 (2018).https://doi.org/10.1016/j.jclepro.2018.02.115
J. X. Chan, J. F. Wong, A. Hassan, et al., Polym. Compos. 41, 395 (2020). https://doi.org/10.1002/pc.25403
P. Kalla, A. Rana, Y. B. Chad, et al., J. Clean. Prod. 87, 726 (2015). https://doi.org/10.1016/j.jclepro.2014.10.038
V. D. Gladun, L. V. Akat’eva, and A. I. Khol’kin, Synthetic Calcium Silicates (IRISBUK, Moscow, 2011) [in Russian].
P. S. Gordienko, S. B. Yarusova, A. V. Kozin, et al., RF Patent No. 2601608, Byull. Izobret., No. 31 (2016).
P. S. Gordienko, V. V. Bagramyan, S. B. Yarusova, A. A. Sarkisyan, G. F. Krysenko, N. V. Polyakova, and Yu. V. Sushkov, Russ. J. Appl. Chem. 85, 1519 (2012). https://doi.org/10.1134/S1070427212100059
Funding
This work was performed as part of a State Task for the Institute of Chemistry, Far Eastern Branch, Russian Academy of Sciences, topic no. 0265-2019-0002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gordienko, P.S., Yarusova, S.B., Buravlev, I.Y. et al. Studying the Kinetics of the Alkaline Processing of Boron Production Wastes under Different Conditions. Russ. J. Phys. Chem. 95, 38–42 (2021). https://doi.org/10.1134/S003602442101009X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602442101009X