Abstract
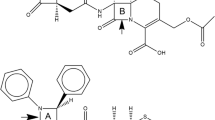
Model molecular systems structurally similar to the transition state of the limiting step of the hydrolysis of cephalosporin antibiotics by the L1 metallo-β-lactamase are studied. The series of fluorinated compounds show that the nature of substituents in thiazine and β-lactam rings have a great impact on the strength of the intramolecular O–H···N hydrogen bond that determines the catalytic parameters in real biological systems. The strengthening or weakening of the O–H···N bond is registered via a quantum topological analysis of the electron density, supplemented with various bonding descriptors’ study. The obtained data are confirmed by the analysis of the vibrational frequency shift relatively to the nonfluorinated compound for the O‒H stretching mode of the carboxylic group involved in the O–H···N bond formation. The absence of the monotonic dependence of the hydrogen bond strength on the donor-acceptor effects of substituents shows that considered bonding descriptors do not provide a complete understanding of the bonding mechanisms in the active center of L1 metallo-β-lactamase.







Similar content being viewed by others
REFERENCES
G. Sliwoski, S. Kothiwale, J. Meiler, and E. W. Lowe, Pharmacol. Rev. 66, 334 (2014). https://doi.org/10.1124/pr.112.007336
S. Mandal, M. Moudgil, and S. K. Mandal, Eur. J. Pharmacol. 625, 90 (2009). https://doi.org/10.1016/j.ejphar.2009.06.065
A. Warshel and M. Levitt, J. Mol. Biol. 103, 227 (1976). https://doi.org/10.1016/0022-2836(76)90311-9
N. Mardirossian and M. Head-Gordon, Mol. Phys. 115, 2315 (2017). https://doi.org/10.1080/00268976.2017.1333644
R. F. W. Bader, Atoms in Molecules. Quantum Theory (Clarendon, Oxford, 1994), p. 432.
M. G. Khrenova and V. G. Tsirelson, Mendeleev Commun. 29 (5), 492 (2019). https://doi.org/10.1016/j.mencom.2019.09.004
M. G. Khrenova, A. V. Tomilko, and V. G. Tsirelson, Mosc. Univ. Chem. Bull. 60, 106 (2019).
R. F. W. Bader and H. Essén, J. Chem. Phys. 80, 1943 (1984). https://doi.org/10.1063/1.446956
L. González et al., J. Chem. Phys. 109, 2685 (1998). https://doi.org/10.1063/1.476868
S. J. Grabowski, T. L. Robinson, and J. Leszczynski, Chem. Phys. Lett. 386, 44 (2004). https://doi.org/10.1016/j.cplett.2004.01.013
M. V. Vener, E. O. Levina, A. A. Astakhov, et al., Chem. Phys. Lett. 638, 233 (2015). https://doi.org/10.1016/j.cplett.2015.08.053
E. Espinosa, I. Alkorta, J. Elguero, et al., J. Chem. Phys. 117, 5529 (2002). https://doi.org/10.1063/1.1501133
D. Cremer, E. Kraka, T. S. Slee, et al., J. Am. Chem. Soc. 105, 5069 (1983). https://doi.org/10.1021/ja00353a036
M. G. Khrenova and A. V. Nemukhin, J. Phys. Chem. B 122, 1378 (2018). https://doi.org/10.1021/acs.jpcb.7b10188
M. W. Crowder, T. R. Walsh, L. Banovic, et al., Antimicrob. Agents Chemother. 42, 921 (1998). https://doi.org/10.1128/AAC.42.4.921
A. Felici and G. Amicosante, Antimicrob. Agents Chemother. 39, 192 (1995). https://doi.org/10.1128/AAC.39.1.192
M. G. Khrenova, A. V. Krivitskaya, and V. G. Tsirelson, New J. Chem. 43, 7329 (2019). https://doi.org/10.1039/C9NJ00254E
A. A. Granovsky, Firefly, Version 8. http://classic.chem.msu.su/gran/firefly/index.html.
C. Adamo and V. Barone, J. Chem. Phys. 110, 6158 (1999). https://doi.org/10.1063/1.478522
S. Grimme, J. Antony, S. Ehrlich, et al., J. Chem. Phys. 132, 154104 (2010). https://doi.org/10.1063/1.3382344
T. Lu and F. Chen, J. Comput. Chem. 33, 580 (2012). https://doi.org/10.1002/jcc.22885
G. Socrates, Infrared and Raman Characteristic Group Frequencies: Tables and Charts (Wiley, New York, 2004).
M. Rozenberg, A. Loewenschuss, and Y. Marcus, Phys. Chem. Chem. Phys. 2, 2699 (2000). https://doi.org/10.1039/B002216K
T. Steiner, Angew. Chem., Int. Ed. Engl. 41, 48 (2002). https://doi.org/10.1002/1521-3773(20020104)41:1<48::AID-ANIE48>3.0.CO;2-U
M. Rozenberg, RSC Adv. 4, 26928 (2014). https://doi.org/10.1039/C4RA03889D
A. V. Afonin, A. V. Vashchenko, and M. V. Sigalov, Org. Biomol. Chem 14, 11199 (2016). https://doi.org/10.1039/C6OB01604A
R. Parthasarathi, V. Subramanian, and N. Sathyamurthy, J. Phys. Chem. A 110, 3349 (2006). https://doi.org/10.1021/jp060571z
I. Mata, I. Alkorta, E. Espinosa, et al., Chem. Phys. Lett. 507, 185 (2011). https://doi.org/10.1016/j.cplett.2011.03.055
Funding
This work was supported by the Russian Science Foundation, project no. 18-74-10056. The research is carried out using the equipment of the shared research facilities of HPC computing resources at Lomonosov Moscow State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have no conflicts of interest to declare.
Additional information
Translated by P. Vlasov
Rights and permissions
About this article
Cite this article
Levina, E.O., Khrenova, M.G. & Tsirelson, V.G. Effect of Substituents in Hydrolyzed Cephalosporins on Intramolecular O–H···N Bond. Russ. J. Phys. Chem. 94, 925–932 (2020). https://doi.org/10.1134/S0036024420050131
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420050131