Abstract
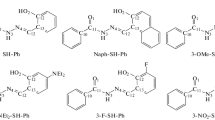
In this paper, two new Schiff bases (L1 and L2) derived from substituted salicylaldehyde and sulfamethoxazole/sulfisoxazole were synthesized. The synthesized structures were elucidated by experimental spectroscopic methods such as FT-IR, 1H-13C NMR, 1H, and 13C shielding tensors, and elemental analysis. The theoretical vibrational modes and nonlinear optical (NLO) properties have been computed by DFT/B3LYP/6-311G(d,p) method. Theoretical 1H and 13C shielding tensors were calculated with GIAO methods in CDCl3 with same level of theory. The results have shown that there is perfect harmony between the calculated parameters and recorded experimental data. The first order hyperpolarizabilities of the new synthesized compounds are 201.79 and 113.14 times larger than that of urea (0.3728 × 10–30 esu), respectively. According to evaluated results, the L1 and L2 present large nonlinear optical activity and are candidate molecules for nonlinear optical applications.




Similar content being viewed by others
REFERENCES
Z. H. Chohan, H. A. Shad, and F. H. Nasim, Appl. Organomet. Chem. 23, 319 (2009).
A. E. Boyd, Diabetes. 37, 847 (1988).
R. Kasımogulları, M. Bülbül, et al., Eur. J. Med. Chem. 45, 4769 (2010).
L. de Luca, S. Ferro, F. M. Damiano, et al., Eur. J. Med. Chem. 71, 105 (2014).
A. D. Wright, M. H. Winterborn, P. J. Forster, J. P. Delamere, G. L. Harrison, and A. R. Bradwell, J. Wilderness Med. 5, 49 (1994).
L. Sun, Y. Wu, Y. Liu, X. Chen, and L. Hu, Bioorg. Med. Chem. Lett. 27, 261 (2017).
H. Yoshino, N. Ueda, et al., J. Med. Chem. 35, 2496 (1992).
C. T. Supuran, Expert Opin. Drug Disc. 12, 61 (2017).
N. Özbek, H. Katırcıoglu, N. Karacan, and T. Baykal, Bioorg. Med. Chem. 15, 5105 (2007).
N. Özbek, S. Alyar, H. Alyar, E. Sahin, and N. Karacan, Spectrochim. Acta, Part A 108, 123 (2013).
F. Akyıldız, S. Alyar, M. T. Bilkan, and H. Alyar, J. Mol. Struct. 1174, 160 (2018).
S. Alyar, T. Şen, Ü. Ö. Özmen, H. Alyar, S. Adem, and C. Şen, J. Mol. Struct. 1185, 416 (2019).
U. Özdemir, N. Karacan, O.S. Senturk, S. Sert, and F. Uğur, Synth. React. Inorg. Metal.-Org. Chem. 34, 1057 (2004).
N. Özbek, S. Alyar, and N. Karacan, J. Mol. Struct. 938, 48 (2009).
A.B. Gündüzalp, Ü. Ö. Özmen, et al., Med. Chem. Res. 23, 3255 (2014).
N. Özbek, G. Kavak, Y. Özcan, S. Ide, and N. Karacan, J. Mol. Struct. 919, 154 (2009).
M. J. Frisch et al., Gaussian 09, Revision B.01 (Gaussian Inc., C.T. Wallingford, 2009).
R. D. Dennington, T. A. Keith, and J. M. Millam, GaussView 5 (Gaussian, Inc., 2008).
M. H. Jamróz, Vibrational Energy Distribution Analysis (VEDA 4, Warsaw, 2004).
M. T. Bilkan, J. Mol. Liq. 238, 523 (2017).
M. T. Bilkan, Russ. J. Phys. Chem. A 92, 1920 (2018).
A. Mahmood, T. Akram, and E. B. de Lima, J. Mol. Struct. 1108, 496 (2016).
F. Blanco, I. Alkorta, and J. Elguero, Magn. Reson. Chem. 45, 797 (2007).
A. M. S. Silva, R. M. S. Sousa, M. L. Jimeno, F. Blanco, I. Alkorta, and J. Elguero, Magn. Reson. Chem. 46, 859 (2008).
H. Soscun, O. Castellano, et al., J. Mol. Struct.: THEOCHEM 592, 19 (2002).
K. S. Thanthiriwatte and K. M. Nalin de Silva, J. Mol. Struct.: THEOCHEM 617, 169 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bilkan, M.T., Alyar, S. & Alyar, H. Experimental Spectroscopic and Theoretical Studies of New Synthesized Sulfonamide Derivatives. Russ. J. Phys. Chem. 94, 143–151 (2020). https://doi.org/10.1134/S0036024420010045
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420010045