Abstract
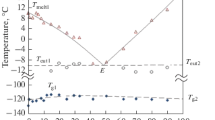
The heat capacity of monoethanolamine (MEA) is measured in the 120–345 K range of temperatures, and changes in the enthalpy, entropy, and reduced Gibbs energy in this range of temperatures are calculated. A significant jump in heat capacity and calculated thermodynamic quantities at 283 K, indicating the melting process, is shown. A jump in the heat capacity at 160 K is observed that is associated with the devitrification of MEA. A change in the temperature dependence in the 200–220 K region is recorded that correlates with the change in the dielectric characteristics of MEA glass in this range of temperatures.

Similar content being viewed by others
REFERENCES
Yu. V. Novakovskaya and M. N. Rodnikova, Russ. J. Inorg. Chem. 59, 1290 (2014). https://doi.org/10.7868/S0044457X14110178
Yu. Ya. Kharitonov, E. G. Khoshabova, M. N. Rodnikova, et al., Dokl. Akad. Nauk SSSR 304, 917 (1989).
M. N. Rodnikova, I. A. Solonina, A. B. Solovei, and T. M. Usacheva, Russ. J. Inorg. Chem. 58, 1501 (2013). https://doi.org/10.7868/S0044457X13120180
Yu. V. Novakovskaya and M. N. Rodnikova, Dokl. Phys. Chem. 467, 60 (2016). https://doi.org/10.7868/S0869565216120148
I. A. Solonina, M. N. Rodnikova, M. R. Kiselev, and A. V. Khoroshilov, Russ. J. Phys. Chem. A 89, 910 (2015). https://doi.org/10.1134/S0036024415050301
Handbook of Physico-Chemical Properties and Environmental Fate for Organic Chemicals (CRC, Boca Raton, FL, 2006), Vol. 4, p. 3236.
O. A. Osipov and V. I. Minkin, Handbook on Dipole Moments (Vyssh. Shkola, Moscow, 1965) [in Russian].
A. B. Razumova, Extended Abstract of Cand. Sci. (Chem.) Dissertation (Yaroslavl, 1994).
Ya. Yu. Akhadov, Dielectric Properties of Pure Liquids (MAI, Moscow, 1999) [in Russian].
V. N. Kartsev, M. N. Rodnikova, V. V. Tsepulin, et al., Zh. Strukt. Khim. 27, 187 (1986).
V. I. Grishchenko, Probl. Kriobiol. 15, 231 (2005).
V. V. Malyshev, G. A. Mil’ner, E. L. Sorkin, and V. F. Shibakin, Prib. Tekh. Eksp., No. 6, 195 (1985).
V. S. Iorish and P. I. Tolmach, Zh. Fiz. Khim. 60, 2583 (1986).
I. A. Solonina, M. A. Vasilyeva, A. Greenbaum (Gutina), Yu. A. Gusev, I. V. Lounev, M. N. Rodnikova, and Yu. Feldman, Russ. J. Phys. Chem. A 90, 117 (2016). https://doi.org/10.1134/S0036024416010283
ACKNOWLEDGMENTS
This work was performed as part of a State Task for the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, in the field of basic scientific research. It was supported by the Russian Foundation for Basic Research, grant no. 16-03-00897.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Boltukhina
Rights and permissions
About this article
Cite this article
Tyurin, A.V., Solonina, I.A. & Rodnikova, M.N. Temperature Dependence of the Heat Capacity of Monoethanolamine. Russ. J. Phys. Chem. 93, 417–420 (2019). https://doi.org/10.1134/S0036024419020286
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419020286