Abstract
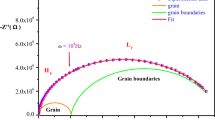
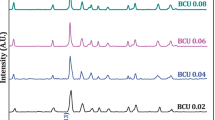
Series of ion conducting system (KI)4 – x‒(CuI)x‒PbI2, x = 0.0‒0.4, have been prepared by solid state reaction method and studied by powder X-ray diffraction, DTA techniques and conductivity measurements. Room temperature XRD reveals the presence of the orthorhombic KPbI3 as the major component in the sample. The X-ray diffraction analysis also confirms the presence of both KI and CuPbI3 in the samples. DTA of x = 0.0 composition shows an endothermic peak at 349°C attributed to the melting of the compound. AC impedance spectroscopy revealed that the contribution of grain is strong over the grain boundary. The addition of CuI content results in the increase of electrical conductivity of the samples. The maximum ionic conductivity σ = 6.33 × 10–1 S cm–1 was shown by x = 0.3 composition at 633 K with lowest activation energy of ~0.11eV in the temperature range of 298‒633 K.





Similar content being viewed by others
REFERENCES
D. A. Keen, J. Phys.: Condens. Matter 14, R819 (2002).
T. K. Mallick, S. Ganguli, and N. Chatterjee, J. Phys. Chem. Solids 45, 661 (1984).
S. Selvasekarapandian and B. Nalini, Solid State Ionics 86–88, 151 (1996).
S. Hull, D. A. Keen, and P. Berastegui, Solid State Ionics 147, 97 (2002).
M. Liu, M. B. Johnston, and H. J. Snaith, Nature (London, U.K.) 501, 395 (2013).
Z. K. Tan, R. S. Moghaddan, M. L. Lai, P. Docampo, R. Higler, F. Deschler, M. Price, A. Sadhanala, L. M. Pazos, D. Credgington, F. Hanusch, T. Bein, H. J. Snaith, and R. H. Friend, Nat. Nanotechnol. 9, 687 (2014).
L. Dou, Y. M. Yang, J. You, Z. Hong, W. H. Chang, G. Li, and Y. Yang, Nat. Commun. 5, 5404 (2014).
Q. Zhang, S. T. Ha, X. Liu, T. C. Sum, and Q. Xiong, Nano Lett. 14, 5995 (2014).
J. W. Brightwell, C. N. Buckley, and B. Ray, Solid State Ionics 15, 61 (1985).
T. A. Kuku and A. M. Salau, Solid State Ionics 25, 1 (1987).
F. Somma, M. Nikl, N. Nitsch, G. Giampaolo, A. R. Phani, and S. Santucci, Thin Solid Films 373, 195 (2000).
O. N. Yunakova, V. K. Miloslavskii, E. N. Kovalenko, and E. V. Ksenfontova, Low Temp. Phys. 38, 943 (2012). doi 10.1063/1.4758777
O. N. Yunakova, V. K. Miloslavskii, and E. N. Kovalenko, Funct. Mater. 20, 59 (2013).
M. Hassan and R. Rafiuddin, Res. Lett. Phys. 2008, 10249402 (2008). doi 10.1155/2008/249402
I. I. Ilyasov, D. G. Barseghov, I. G. Berikashili, et al., J. Inorg. Chem. 14, 1484 (1969).
O. N. Yunakova, V. K. Miloslavsky, E. N. Kovalenko, and V. V. Kovalenko, Low Temp. Phys. 40, 690 (2014).
D. M. Trots and S. V. Myagkota, J. Phys. Chem. Solids 69, 2520 (2008).
J. P. C. Evangelista, Th. Chellapa, A. C. F. Coriolano, V. J. Fernandes, Jr., L. D. Souza, and A. S. Araujo, Fuel Process. Technol. 104, 90 (2012).
T. S. Ripolles, K. Nishinake, Y. Ogomi, Y. Miyata, and Sh. Hayase, Sol. Energy Mater. Sol. Cells 144, 532 (2016).
Rafiuddin and M. Hassan, Solid State Commun. 144, 293 (2007).
S. Sultana and Rafiuddin, J. Alloys Compd. 509, 9842 (2011).
G. H. Pereira, R. H. R. Castro, D. Z. de Floria, E. N. S. Muccillo, and D. Gouvea, Mater. Lett. 59, 1195 (2005).
D. C. Onwudiwe, T. Arfin, and C. A. Strydom, Electrochim. Acta 127, 283 (2014).
V. Thangadurai, H. Kaack, and W. J. F. Weppner, J. Am. Ceram. Soc. 86, 437 (2003).
P. Knauth, J. Electroceram. 5, 111 (2000).
V. G. Ponomareva and G. V. Lavrova, Solid State Ionics 145, 197 (2001).
N. F. Uvarov, B. B. Bohonov, V. P. Isupov, and E. F. Hairetdinov, Solid State Ionics 74, 15 (1994).
R. B. Beeken, J. C. Faludi, W. M. Schreir, and J. M. Tritz, Solid State Ionics 719, 154 (2002).
R. B. Beeken and E. M. Beeken, Solid State Ionics 463, 136 (2000).
R. B. Beeken, W. L. Jetzer, and D. R. Smith, Solid State Ionics 176, 70 (1994).
ACKNOWLEDGMENTS
The authors are indebted to the Chairman, Department of Chemistry for providing all facilities which were recommended for the research work and UGC, New Delhi, India for the commercial support. We are also thankful to the Applied Physics Department, AMU Aligarh for making XRD technique available for structural analysis of the samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Suhail Iqbal Wani, Rafiuddin Structural, Thermal, and Electrical Behavior of Cu-Substituted KPbI3 Ternary Compound. Russ. J. Phys. Chem. 92, 2811–2816 (2018). https://doi.org/10.1134/S0036024418130332
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024418130332