Abstract
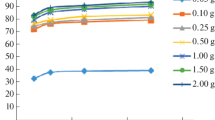
Data on the effect temperature has on the kinetics of the removal of Co2+ and Ni2+ ions under static conditions by a sorbent based on a derivative of phytic acid fabricated from rice production waste are presented. It is shown that when the temperature is raised from 20 to 60°C, the sorption capacity of the sorbent based on phytic acid increases over the period of sorption and within 180 min reaches values of 1.4 mmol g−1 for Co2+ ions and 1.3 mmol g−1 for Ni2+ ions. It is established that for the investigated range of temperatures, order n of the sorption of Co2+ and Ni2+ ions is <1, which characterizes the reactions accompanied by diffusion processes. It is found that the process of removal of Co2+ and Ni2+ ions is characterized with low activation energy (20.74 kJ mol−1 for Co2+ ions and 14.2 kJ mol−1 for Ni2+ ions). It is also demonstrated that the sorption process in the considered time frame is best described by a kinetic model of a pseudo-second order, as is indicated by respective correlation coefficients.
Similar content being viewed by others
References
K. A. Saburov and Kh. M. Kamilov, Chem. Nat. Compd. 25, 695 (1989).
L. G. Barrientos and P. P. N. Murthy, Carbohydr. Res. 296, 39 (1996).
V. Raboy, Phytochemistry 64, 1033 (2003).
E. Vasca, S. Materazzi, T. Caruso, et al., Anal. Bioanal. Chem. 373, 173 (2002).
M. R. Truter and M. E. Tate, J. Chem. Soc. B 697 9, 70 (1970).
F. Crea, C. de Stefano, D. Milea, et al., Coord. Chem. Rev. 252, 1108 (2008).
M. D. Mashkovskii, Drugs (Novaya Volna, Moscow, 2009) [in Russian].
A. Bebort-Brigaud, C. Dange, N. Fauconnier, et al., J. Inorg. Biochem. 75, 71 (1999).
H. Perrsson, M. Turk, M. Nyman, et al., J. Agric. Food Chem. 46, 3194 (2008).
C. de Stefano, D. Milea, N. Porcino, et al., J. Agric. Food Chem. 54, 1459 (2006).
G. T. Tsao, Y. Zheng, J. Lu, and C. S. Gong, Appl. Biochem. Biotechnol. 63–65, 731 (1997).
F. Iemma, G. Cirillo, U. G. Spizzirri, et al., Eur. Polym. J. 44, 1183 (2008).
U. Ulusoy and S. Şimşek, J. Hazard. Mater. 127, 163 (2005).
M. Ruyter-Hooley, A. C. Larsson, B. B. Johnson, et al., J. Colloid Interface Sci. 474, 159 (2016).
R. Li, L. Liu, F. Yang, et al., J. Hazard. Mater. 280, 20 (2014).
N. V. Makarenko, S. B. Yarusova, Yu. A. Azarova, et al., Vestn. DVO RAN, No. 4, 94 (2015).
L. A. Zemnukhova, S. B. Yarusova, N. V. Makarenko, et al., in Ecological Problems of Nature Management and Environment Protection in the Asia-Pacific Region: Habitats, Their Protection and Restoration (Dal’nauka, VGUES, Vladivostok, 2016) [in Russian].
L. G. Kolzunova, L. A. Zemnukhova, G. A. Fedorishcheva, L. N. Kurilenko, and V. I. Sergienko, Russ. J. Appl. Chem. 73, 1726 (2000).
V. V. Boldyrev, Study Methods of Kinetics of Thermal Decomposition of Solids (Tomsk. Gos. Univ., Tomsk, 1958) [in Russian].
N. J. Coleman, D. S. Brassington, A. Raza, et al., Waste Manage. 26, 260 (2006).
Y. S. Ho and G. McKay, Process Biochem. 34, 451 (1999).
V. A. Reutov, Mechanical Processes, Manual for Laboratory Practice (Dal’nevost. Univ., Vladivostok, 2005) [in Russian].
L. M. Kovba and V. K. Trunov, X-ray Phase Analysis (Mosk. Gos. Univ., Moscow, 1976) [in Russian].
N. V. Makarenko, U. V. Kharchenko, A. B. Slobodyuk, and L. A. Zemnukhova, Khim. Rast. Syr’ya, No. 3, 255 (2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yarusova, S.B., Makarenko, N.V., Gordienko, P.S. et al. Effect of Temperature on the Kinetics of Sorption of Co2+ and Ni2+ Ions by a Sorbent Based on an Inositol Hexaphosphoric Acid Derivative. Russ. J. Phys. Chem. 92, 559–564 (2018). https://doi.org/10.1134/S0036024418030354
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024418030354