Abstract
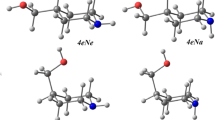
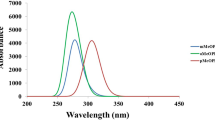
Quantum-chemical calculations of the 3-(hydroxymethyl)piperidine molecule conformers were performed at the B3LYP/6-31+G** level of theory, and four most stable conformations with different relative orientation of CH2OH and N–H groups were determined. The optimized structures, vibration frequencies, and band intensities in the spectra of the conformers were obtained. The conformational equilibria of the most stable rotational isomers in solvents of different polarity was studied within the polarizable continuum model. According to the results of calculations, the conformational equilibrium in solution is substantially changed on varying the solvent polarity. This conclusion was confirmed by comparison with IR absorption spectra of 3-(hydroxymethyl)piperidine solutions in carbon tetrachloride in the region of ОН-stretchings.
Similar content being viewed by others
References
Dictionary of Organic Compounds, 6th ed. (Chapman Hall, CRC, London, 1995), p. 5383.
E. L. Eliel, N. L. Allinger, S. J. Angyal, and G. A. Morrison, Conformational Analysis (Wiley, New York, 1965), p.245.
N. L. Allinger, J. G. D. Carpenter, and F. M. Karkowski, Tetrahedron Lett., 3345 (1964).
F. A. L. Anet and I. Yavari, J. Am. Chem. Soc. 99, 2794 (1977).
J. E. Parkin, P. J. Buckley, and C. C. Coin, J. Mol. Spectrosc. 89, 465 (1981).
R. W. Baidock and A. R. Katritzky, Tetrahedron Lett., 1159 (1968).
D. W. Scott, J. Chem. Thermodyn. 3, 649 (1979).
M. T. Gulluoglu, Y. Erdogdu, and S. Yurdakul, J. Mol. Struct. 834–836, 540 (2007).
G. Gundersen and D. W. Rankin, Acta Chem. Scand. A 37, 865 (1983).
A. Goldblum, O. Deeb, and G. H. Loew, J. Mol. Struct.: THEOCHEM 207, 1 (1990).
www.chemcraftprog.com. Version 1.8, build486.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 03, Rev. C.02 (Gaussian Inc., Wallingford, CT, 2004).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 09, Rev. D.01 (Gaussian Inc., Wallingford, CT, 2013).
V. A. Rassolov, M. A. Ratner, J. A. Pople, et al., J. Comput. Chem. 22, 976 (2001).
F. Jensen, Introduction to Computational Chemistry (Wiley, Chichester, UK, 1999), p.446.
Y. Yang, M. N. Weaver, K. M. Weaver, et al., J. Phys. Chem. A 113, 9843 (2009).
Y. Zhao and D. G. Truhlar, Theor. Chem. Acc. 120, 215 (2008).
V. Barone and M. Cossi, J. Phys. Chem. A 102, 1995 (1998).
M. Cossi, N. Rega, G. Scalmani, et al., J. Comput. Chem. 24, 669 (2003).
A. Frisch, Gaussian 09: User’s Reference (Gaussian Inc., Wallingford, CT, 2009).
J. B. Foresman and E. Frisch, Exploring Chemistry with Electronic Structure Methods (Gaussian, Pittsburgh, PA, 1996).
I. V. Kochikov and G. M. Kuramshina, Vestn. Mosk. Univ., Ser. Khim. 26, 354 (1985).
I. V. Kochikov, G. M. Kuramshina, Yu. A. Pentin, et al., Zh. Fiz. Khim. 64, 3393 (1990).
D. Vedal, O. Ellestad, and P. Klaboe, Spectrochim. Acta A 32, 877 (1976).
D. Lin-Vien, N. B. Colthup, W. G. Fateley, et al., The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules (Elsevier, Amsterdam, 1991), p. 164.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.Ya. Korneichuk, V.M. Senyavin, G.M. Kuramshina, 2017, published in Zhurnal Fizicheskoi Khimii, 2017, Vol. 91, No. 2, pp. 354–360.
Rights and permissions
About this article
Cite this article
Korneichuk, A.Y., Senyavin, V.M. & Kuramshina, G.M. Conformation equilibrium of 3-(hydroxymethyl)piperidine in solvents with different polarity. Russ. J. Phys. Chem. 91, 351–357 (2017). https://doi.org/10.1134/S0036024417020170
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024417020170