Abstract
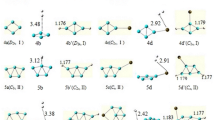
Calcium borohydride is widely studied as a hydrogen storage material. However, investigations on calcium borohydride from a cluster perspective are seldom found. The geometric structures and binding energies of [Ca(BH4)2] n (n = 1–4) clusters are determined using density function theory (DFT). For the most stable structures, vibration frequency, natural bond orbital (NBO) are calculated and discussed. The charge transfer from (BH4) to Ca was observed. Meanwhile, we also study the LUMO–HOMO gap (E g) and the B–H bond dissociation energies (BDEs). [Ca(BH4)2]3 owns higher E g, revealing that trimer is more stable than the other forms. Structures don’t change during optimization after hydrogen radical removal, showing that calcium borohydride could possibly be used as a reversible hydrogen storage material. [Ca(BH4)2]4 has the smallest dissociation energy suggesting it releases hydrogen more easily than others.
Similar content being viewed by others
References
I. Dovgaliuk, V. Ban, Y. Sadikin, et al., J. Phys. Chem. C 118, 145 (2014).
B. L. Chittari and S. P. Tewari, Phys. Status Solidi B 251, 898 (2014).
H. Chu, S. Qiu, L. Sun, et al., J. Renew. Sustain. Energy 6, 013105 (2014).
Y. Filinchuk, B. Richter, T. R. Jensen, et al., Angew. Chem. Int. Ed. 50, 11162 (2011).
W. Grochala and P. P. Edwards, Chem. Rev. 35, 1283 (2004).
E. Hazrati, G. Brocks, and G. A. de Wijs, J. Phys. Chem. C 118, 5102 (2014).
L. George and S. K. Saxena, Int. J. Hydrogen Energy 35, 5454 (2010).
P. Zhang, B. Xu, X. Li, et al., Int. J. Hydrogen Energy 39, 17144 (2014).
E. Rönnebro, Curr. Opin. Solid State M 15, 44 (2011).
M. D. Riktor, M. H. Sørby, K. Chlopek, et al., J. Mater. Chem. 17, 4939 (2007).
Y. Filinchuk, E. Ronnebro, and D. Chandra, Acta Mater. 57, 732 (2009).
Purusottam Jena et al., Tech. Report no. 0704-0188 (2014).
D. M. Liu, C. Gao, Z. X. Qian, et al., Int. J. Hydrogen Energy 38, 3291 (2013).
S. Orimo, Y. Nakamori, J. R. Eliseo, et al., Chem. Rev. 107, 4111 (2007).
C. Paduani, M. M. Wu, M. Willis, et al., J. Phys. Chem. A 115, 10237 (2011).
M. Fichtner, J. Engel, O. Fuhr, et al., Inorg. Chem. 42, 7060 (2003).
R. Mohtadi, P. Sivasubramanian, S. J. Hwang, et al., Int. J. Hydrogen Energy 37, 2388 (2012).
X. Chen, F. Yuan, Y. Tan, et al., J. Phys. Chem. C 116, 21162 (2012).
N. Bindzus, F. Cargnoni, B. B. Iversen, et al., J. Phys. Chem. C 117, 2308 (2013).
L. H. Rude, M. Corno, P. Ugliengo, et al., J. Phys. Chem. C 116, 20239 (2012).
D. A. Knight, R. Zidan, R. Lascola, et al., J. Phys. Chem. C 117, 19905 (2013).
H. W. Li, E. Akiba, and S. Orimo, J. Alloys Compd. 580, S292 (2013).
S. Li, M. Willis, and P. Jena, J. Phys. Chem. C 114, 16849 (2010).
S. H. Lee, V. R. Manga, and Z. K. Liu, Int. J. Hydrogen Energy 35, 6812 (2010).
Y. Guo, J. Jia, X. H. Wang, et al., Chem. Phys. 418, 22 (2013).
D. M. F. Santos and C. A. C. Sequeira, Renew. Sust. Energy Rev. 15, 3980 (2011).
P. Martelli, R. Caputo, A. Remhof, et al., J. Phys. Chem. C 114, 7173 (2010).
O. Zavorotynska, M. Corno, A. Damin, et al., J. Phys. Chem. C 115, 18890 (2011).
D. S. Sholl and K. C. Kim, J. Phys. Chem. C 114, 678 (2009).
Y. Yang, X. Wu, C. Liu, et al., Chem. Phys. 443, 45 (2014).
J. H. Kim, S. A. Jin, J. H. Shim, et al., Scripta Mater. 58, 481 (2008).
E. H. Majzoub and E. Rönnebro, J. Phys. Chem. C 113, 3352 (2008).
X. H. Li and X. H Ju, Comput. Theor. Chem. 1025, 46 (2013).
M. Fichtner, K. Chlopek, M. Longhini, et al., J. Phys. Chem. C 112, 11575 (2008).
T. Noritake, M. Aoki, M. Matsumoto, et al., J. Alloy. Compd. 491, 57 (2010).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B 37, 785 (1988).
T. Yanai, D. P. Tew, and N. C. Handy, Chem. Phys. Lett. 393, 51 (2004).
S. R. Pruitt, S. S. Leang, P. Xu, et al., Comput. Theor. Chem. 1021, 70 (2013).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 09, Revision C.01 (Gaussian Inc., Wallingford, CT, USA, 2009).
K. Miwa and M. Aoki, Phys. Rev. B 74, 155122 (2006)
S. F. Parker, Coord. Chem. Rev 254, 215 (2010)
S. J. Blanksby and G. B. Ellison, Acc. Chem. Res. 36, 255 (2003).
C. B. Lingam and S. P. Tewari, Comput. Theor. Chem. 1020, 151 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Han, C., Dong, Y., Wang, B. et al. [Ca(BH4)2] n clusters as hydrogen storage material: A DFT study. Russ. J. Phys. Chem. 90, 1997–2005 (2016). https://doi.org/10.1134/S0036024416100071
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024416100071