Abstract
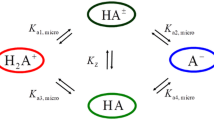
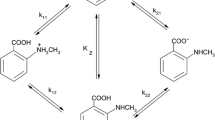
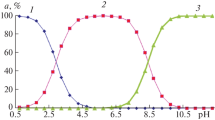
The acid dissociation constants of form pK 1 = 7.34 ± 0.01, pK 2 = 7.84 ± 0.01, pK 3 = 8.77 ± 0.01, pK 4 = 9.49 ± 0.01, and pK 5 = 10.70 ± 0.02 of cationic amikacin are determined by pH-metric titration at 25°C against the background of 0.1 mol/L KNO3. K 1, K 2, K 3, and K 4 correspond to the dissociation of protons coordinated to amino groups, while K 5 characterizes the dissociation of the hydroxyl hydrogen atom, testifying to the amphoteric character of amikacin molecules. Applying density functional theory (DFT) with the B3LYP hybrid functional and the 6-311G**++ basis set, the partial charges on the atoms of an amikacin molecule are calculated. It is concluded that the dissociation of H(55)hydrogen atom occurs with a greatest partial charge of +0.53631.
Similar content being viewed by others
References
S. N. Kozlov and L. S. Strachunskii, Modern Antimicrobial Chemotherapy (Med. Inform. Agentstvo, Moscow, 2009) [in Russian].
J. L. Houghton, K. D. Green, W. Chen, and S. Garneau-Tsodikova, Chem Bio Chem 11, 880 (2010).
P. Dozzo and H. E. Mozer, Expert Opin. Ther. Patents 20, 1321 (2010).
M. D. Mashkovskii, Medicines, 16th ed. (Novaya Volna, Moscow, 2012) [in Russian].
A. Krezel, W. Szczepanic, M. Swiatek, and M. Jezowska-Bojczuk, Bioorg. Med. Chem. 12, 4075 (2004).
E. Gaggelli, N. Gaggelli, A. Maccotta, et al., Spectrochim. Acta, Part A 51, 1959 (1995).
J. Cox and E. H. Serpersu, Biochemistry 36, 2353 (1997).
R. S. Kane, P. T. Glink, R. G. Chapman, et al., Anal. Chem. 73, 4028 (2001).
wwwzipgomelby/i160
wwwhyperquadcouk/HQ2013htm
A. D. Bochevarov, E. Harder, T. F. Hughes, et al., Int. J. Quantum Chem. 113, 2110 (2013).
wwwschrodingercom/products/
http://openmopacnet/MOPAC2012html
R. F. Jameson and M. F. Wilson, J. Chem. Soc., Dalton Trans., No. 23, 2607 (1972).
P. M. Sabale, P. Kaur, Y. Patel, et al., J. Chem. Pharm. Res. 4, 4921 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.G. Alekseev, E.V. Markova, 2016, published in Zhurnal Fizicheskoi Khimii, 2016, Vol. 90, No. 3, pp. 380–385.
Rights and permissions
About this article
Cite this article
Alekseev, V.G., Markova, E.V. Constants of acid‒base equilibria in an aqueous amikacin aminoglycoside solution at 298 K. Russ. J. Phys. Chem. 90, 586–591 (2016). https://doi.org/10.1134/S0036024416020035
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024416020035