Abstract
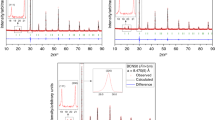
The thermodynamic properties and oxygen stoichiometry of Ba2Cu3O5 + δ are studied by means of the electromotive force (EMF) with a fluoride electrolyte, dissolution calorimetry, and thermogravimetry. It is shown that the temperature dependence of the Gibbs energy of the formation of barium cuprate from simple oxides and oxygen in the temperature range of 860–1120 K can be described by the polynomial Δf, ox G ∘(Ba2Cu3O5 + δ) ± 0.1 (kJ/mol) = −291.78 + 1.127T − 0.13207 TlnT (kJ/mol).
Similar content being viewed by others
References
J. C. Thompson, J. D. F. Gerald, R. L. Withers, et al., Mater. Res. Bull. 24, 505 (1989).
L. A. Klinkova, I. V. Soikina, I. I. Zver’kova, et al., Neorg. Mater. 25, 2033 (1989).
L. A. Klinkova, I. V. Soikina, and I. V. Romanenko, Zh. Neorg. Khim. 35, 446 (1990).
G. V. Bazuev and V. N. Krasil’nikov, Zh. Neorg. Khim. 36, 2195 (1991).
T. K. Jondo, S. Phok, Ph. Galez, and J.-L. Jorda, Crystal Eng. 5, 419 (2002).
S. F. Pashin, E. V. Antipov, L. M. Kovba, and Yu. Ya. Skolis, Sverkhprovodimost’: Fiz., Khim., Tekh. 2 (7), 102 (1989).
W. Wong-Ng and L. P. Cook, Powder Diffract. 9, 280 (1994).
E. L. Brosha, F. H. Garzon, I. D. Raistrick, and P. K. Davies, J. Solid State Chem. 122, 176 (1996).
Yu. Ya. Skolis, S. F. Pashin, and M. L. Kovba, Sverkhprovodimost’: Fiz., Khim., Tekh. 3, 2792 (1990).
A. F. Maiorova, S. N. Mudretsova, M. L. Kovba, A. S. Monaenkova, and A. A. Popova, Physica C 218, 137 (1993).
G. F. Voronin, Pure Appl. Chem. 64, 27 (1992).
G. F. Voronin and S. A. Degterov, J. Solid State Chem. 110, 50 (1994).
V. A. Lysenko, Inorg. Mater. 34, 910 (1998).
V. A. Lysenko, Russ. J. Phys. Chem. A 74, 1237 (2000).
T. B. Lindemer and E. D. Specht, Physica C 255, 81 (1995).
L. A. Klinkova, V. I. Nikolaichik, N. V. Barkovskii, and V. K. Fedotov, J. Solid State Chem. 184, 1834 (2011).
M. L. Kovba, A. V. Saushev, L. P. Ogorodova, and I. A. Uspenskaya, Russ. J. Phys. Chem. A 79, 34 (2005).
Yu. Ya. Skolis, M. L. Kovba, and L. A. Khramtsova, Zh. Fiz. Khim. 65, 1070 (1991).
A. F. Vorob’ev, A. S. Monaenkova, and E. B. Pashlova, Zh. Obshch. Khim. 48, 6 (1978).
G. C. Fitgibbon, E. J. Huber, and C. E. Holley, Jr., J. Chem. Thermodyn. 5, 577 (1973).
A. S. Monayenkova, A. F. Vorob’ev, A. A. Popova, and L. A. Tiphlova, J. Chem. Thermodyn. 34, 1777 (2002).
A. S. Monaenkova, A. A. Popova, L. A. Tiflova, et al., Russ. J. Phys. Chem. A 70, 551 (1996).
E. H. P. Cordfunke, R. J. M. Koning, and W. Ouweltjes, J. Chem. Thermodyn. 22, 991 (1990).
L. Nunez, G. Pilcher, and H. A. Skinner, J. Chem. Thermodyn. 1, 31 (1969).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.L. Kovba, L.A. Tiflova, S.Ya. Istomin, Yu.Ya. Skolis, A.S. Monaenkova, 2014, published in Zhurnal Fizicheskoi Khimii, 2014, Vol. 88, No. 3, pp. 387–391.
Rights and permissions
About this article
Cite this article
Kovba, M.L., Tiflova, L.A., Istomin, S.Y. et al. Thermodynamic properties and oxygen stoichiometry of Ba2Cu3O5 + δ . Russ. J. Phys. Chem. 88, 372–376 (2014). https://doi.org/10.1134/S0036024414030133
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024414030133