Abstract
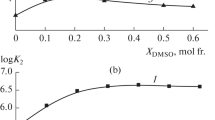
Stability constants of nickel(II) glycylglycinate complexes in aqueous solutions of dimethylsulfoxide of variable composition (from 0.00 to 0.60 mole fractions DMSO) are determined according to potentiometry at 298.15 K and an ionic strength of 0.1 M (NaClO4). It is determined that with a rise in the concentration of an organic cosolvent in solution, the stability of nickel(II) complexes with glycylglycinate ion on the whole increases, but the logK stability = f(X DMSO) dependences are of a critical character with a maximum of 0.3 mole fractions DMSO. It is demonstrated that the rise in the stability of complexes is related to the destabilization of ligands in the low concentration range of the organic component, while the presence of a maximum is due to the different dynamics of the solvation contributions from reagents during changes in the Gibbs energy of reaction.
Similar content being viewed by others
References
M. Lim and G. Nancollas, Inorg. Chem. 10, 1957 (1971).
V. A. Isaeva, V. V. Naumov, and V. A. Sharnin, Russ. J. Coord. Chem. 35, 868 (2009).
V. V. Naumov, V. A. Isaeva, and V. A. Sharnin, Russ. J. Inorg. Chem. 56, 1139 (2011).
V. A. Borodin, V. P. Vasil’ev, and E. V. Kozlovskii, Zh. Neorg. Khim. 31, 10 (1986).
V. V. Naumov, V. A. Isaeva, V. A. Sharnin, and E. N. Kuzina, Russ. J. Phys. Chem. A 85, 1752 (2011).
C. Monk, Trans. Faraday Soc. 47, 285 (1951).
N. V. Petrov, V. S. Nabokov, B. V. Zhadanov, et al., Zh. Fiz. Khim. 50, 2208 (1976).
E. Tipping and H. Scinner, J. Chem. Soc., Faraday Trans. 1 68, 1764 (1972).
M. Lim and G. Nancollas, Inorg. Chem. 10, 1957 (1971).
V. A. Sharnin, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol. 48(7), 44 (2005).
V. A. Isaeva, V. A. Sharnin, and V. A. Shormanov, Russ. J. Phys. Chem. A 72, 1985 (1998).
V. A. Isaeva, V. A. Sharnin, V. A. Shormanov, and S. F. Ledenkov, Russ. J. Phys. Chem. A 70, 1232 (1996).
V. A. Isaeva, V. A. Sharnin, and V. A. Shormanov, Russ. J. Coord. Chem. 25, 852 (1999).
V. A. Isaeva, S. F. Ledenkov, V. A. Sharnin, and V. A. Shormanov, Koord. Khim. 21, 396 (1995).
E. N. Tsurko, N. V. Bondarev, T. M. Shikhova, and E. V. Khrebto, Russ. J. Coord. Chem. 31, 291 (2005).
Zh. F. Gesse, V. A. Isaeva, and V. A. Sharnin, Russ. J. Phys. Chem. A 84, 329 (2010).
V. V. Naumov, V. A. Isaeva, and V. A. Sharnin, in Proceedings of the 6th Conference of Young Scientists on Theoretic and Experimental Chemistry of Liquid Phase Systems (Ivanovo, 2011), p. 98.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.V. Naumov, V.A. Isaeva, Yu.A. Kovaleva, V.A. Sharnin, 2013, published in Zhurnal Fizicheskoi Khimii, 2013, Vol. 87, No. 7, pp. 1160–1163.
Rights and permissions
About this article
Cite this article
Naumov, V.V., Isaeva, V.A., Kovaleva, Y.A. et al. Stability of nickel(II) glycylglycinate complexes in aqueous solutions of dimethylsulfoxide at 298.15 K. Russ. J. Phys. Chem. 87, 1135–1137 (2013). https://doi.org/10.1134/S0036024413070224
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024413070224