Abstract
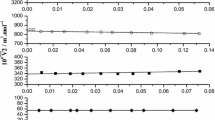
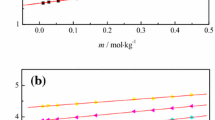
Densities, viscosities, and surface tensions have been measured for n-octanol-phosphoric acid solutions in the range from x 1 = 0 to 0.0266 and temperature from 293.15 to 333.15 K at atmospheric pressure. The coefficient of thermal expansion, excess molar volumes and deviations of surface tension are calculated from the experimental data. The excess molar volumes and deviations of surface tensions are fitted to use the Redlich-Kister polynomial equation. According to the experimental data, the measured viscosities are well fitted to regression equation under the correlating temperature and mass fraction of phosphoric acid. The experimental and calculated thermodynamic properties can be used in research on the nature of mixing behavior of the solutions for molecular models and industrial applications.
Similar content being viewed by others
References
H. Ahmed, H. Diamonta, C. Chaker, and R. Abdelhamid, Sep. Purif. Technol. 55, 212 (2007).
M. Y. Huang, B. H. Zhong, and J. Li, J. Chem. Eng. 53, 2030 (2008).
B. Mnica, D. Gramajo, and H. N. Slimo, J. Chem. Eng. 44, 430 (1999).
Q. L. Tian and H. Z. Liu, J. Chem. Eng. Data 52, 892 (2007).
X. Z. Shao, J. S. Wu G. Q. Zhang, and L. S. Wang, J. Chem. Eng. Data 53, 1012 (2008).
S. Fang, C. X. Zhao, and H. H. He, J. Chem. Eng. Data 53, 2244 (2008).
A. Keshav, S. Chand, and K. L. Isewar, J. Chem. Eng. Data 53, 1424 (2008).
F. Smagghe, J. Xu, and M. Faizal, J. Chem. Eng. Data 37, 24 (1992).
Y. Zhang and M. Muhammed, Solvent Extr. Ion Exch. 6, 973 (1988).
S. Khorfan, O. Shino, and A. Wahoud, Period. Polytech., Chem. Eng. 45, 140 (2001).
K. D. Kurnia, B. Ariwahjoedi, M. I. A. Mutalib, and T. Murugesan, J. Solution Chem. 40, 470 (2011).
M. I. Amin, M. M. Ali, H. M. Kamal, A. M. Youssef, and M. A. Akl, Hydrometallurgy 105, 115 (2010).
U. Domanska and M. Laskowska, J. Solution Chem. 38, 779 (2009).
X. B. Jiang, Y. Y. Zhao, B. H. Hou, M. J. Zhang, and Y. Bao, J. Chem. Eng. Data 56, 206 (2011).
Y. C. Kao and C. H. Tu, J. Chem. Thermodyn. 43, 216 (2011).
J. G. Li, Y. F. Hu, S. F. Sun, Y. S. Liu, and Z. C. Liu, J. Chem. Thermodyn. 42, 904 (2010).
F. Mei, W. Qin, and Y. Y. Dai, J. Chem. Eng. Data 47, 941 (2002).
D. S. Zheng, J. Li, K. Zhou, J. H. Luo, and Y. Jin, J. Chem. Eng. Data 55, 59 (2010).
Compilation Group of Common Chemistry Handbook, Common Chemistry Handbook (Geology, Beijing, 1997).
N. L. Cheng and S. W. Hu, Solvents Handbook, 1st ed. (Chem. Eng., Beijing, 1986).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Ye, C.W., Li, J. Density, viscosity, and surface tension of n-octanol-phosphoric acid solutions in a temperature range 293.15–333.15 K. Russ. J. Phys. Chem. 86, 1515–1521 (2012). https://doi.org/10.1134/S0036024412100263
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024412100263