Abstract
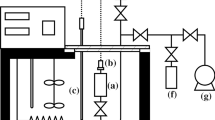
Saturation vapor pressures and vaporization enthalpies of ethylene glycol and C1–C5 carboxylic acid disubstituted esters of normal and branched structures are determined by the transfer method in the temperature range of 295 to 327 K. Dependences of vaporization enthalpies versus the number of carbon atoms in a molecule and the retention indices are determined. An analysis of existing calculation schemes is given to help predict the vaporization enthalpy of the compounds under study.
Similar content being viewed by others
References
A. S. Maslakova, E. L. Krasnykh, and S. V. Levanova, Zh. Fiz. Khim. 84, 214 (2010) [Russ. J. Phys. Chem. A 84, 163 (2010)].
C. A. Taylor and Wm. H. Rinkenbach, J. Am. Chem. Soc. 48, 1305 (1926).
S. Ji, L. Wan, and Z. Fan, Water, Air, Soil Pollut. 132, 347 (2001).
C.-Y. Lin and J.-C. Huang, Ocean Eng. 30, 1699 (2003).
R. M. Stephenson and S. Malanovski, Handbook of the Thermodynamics of Organic Compounds (Elsevier, New York, 1987).
K. Kusano and I. Wadso, Acta Chem. Scand. 24, 2037 (1970).
S. O. Nilsson and I. Wadso, J. Chem. Thermodyn. 18, 673 (1986).
S. P. Verevkin, V. N. Emel’yanenko, A. V. Toktonov, et al., Ind. Eng. Chem. Res. 48, 7388 (2009).
A. S. Leol’ko, E. L. Krasnykh, S. V. Levanova, and I. K. Kukushkin, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol. 50(4), 125 (2007).
A. S. Leol’ko, E. L. Krasnykh, and S. V. Levanova, Zh. Anal. Khim. 64, 1154 (2009) [J. Anal. Chem. 64, 1126 (2009)].
S. P. Verevkin, E. L. Krasnykh, T. V. Vasiltsova, and A. Heintz, J. Chem. Eng. Data 48, 591 (2003).
S. V. Lipp, E. L. Krasnykh, and S. V. Levanova, Zh. Fiz. Khim. 82, 2250 (2008) [Russ. J. Phys. Chem. A 82, 2025 (2008)].
V. P. Spiridonov and A. A. Lopatkin, Mathematical Processing of Physicochemical Data (Mosk. Gos. Univ., Moscow, 1970) [in Russian].
J. Chickos and W. Acree, Jr., J. Phys. Chem. Ref. 23, 519 (2003).
N. Cohen, J. Chem. Ref. Data 25, 1411 (1996).
M. Ducros, J. F. Gruson, and H. Sannier, Thermochim. Acta 36, 39 (1980).
Yu. A. Lebedev and E. A. Miroshnichenko, in Thermochemistry of Vapor Formation of Organic Substances (Nauka, Moscow, 1981) [in Russian].
E. S. Domalski and E. D. Hearing, J. Phys. Chem. Ref. Data 22, 816 (1993).
D. Dalmazzone, A. Salmon, and S. Guella, Fluid Phase Equilib. 242, 29 (2006).
J. Marrero and R. Gani, Fluid Phase Equilib. 183–184, 183 (2001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.S. Maslakova, E.L. Krasnykh, S.V. Levanova, 2011, published in Zhurnal Fizicheskoi Khimii, 2011, Vol. 85, No. 10, pp. 1822–1827.
Rights and permissions
About this article
Cite this article
Maslakova, A.S., Krasnykh, E.L. & Levanova, S.V. Saturation vapor pressures and vaporization enthalpies of esters of ethylene glycol and lower carboxylic acids. Russ. J. Phys. Chem. 85, 1695–1700 (2011). https://doi.org/10.1134/S0036024411100116
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024411100116