Abstract
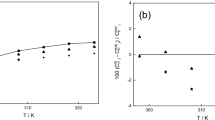
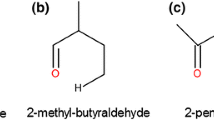
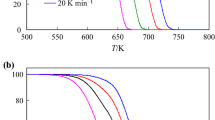
Revision of the experimental data on α-pinene thermal isomerization attained in supercritical ethanol allowed us to expand the reaction scheme, which includes now six main products and eleven reversible reactions. The equilibrium constants of every reaction (K T, j and K Φ, j) were calculated to allow for reversibility of reactions. The thermochemical data of the pure compounds required to calculate constants K T, j and K Φ, j (standard enthalpy and entropy of formation Δf H° (298.15 K), Δf S° (298.15 K), heat capacity C p (T), critical parameters T cr and p cr, boiling point T b, and the acentric factor ω) were preliminary estimated using the empirical Joback and Benson methods. A kinetic model based on the new expanded scheme of reversible reactions was successfully identified and its kinetic parameters k j (600 K) and E j were determined. Detailed examination of the new kinetic model allowed us to refine the generally accepted mechanism of α-pinene thermal isomerization and to distinguish additional features of the multistep process.
Similar content being viewed by others
References
A. Yermakova, A. M. Chibiryaev, I. V. Kozhevnikov, et al., Chem. Eng. Sci. 62, 2414 (2007).
S. G. Traynor, K. J. Crowley, and W. Cocker, Tetrahedron 34, 2783 (1978).
K. G. Joback and R. C. Reid, Chem. Eng. Commun. 57, 233 (1987).
B. I. Lee and M. G. Kesler, AIChE J. 21, 510 (1975).
D. Ambrose and J. Walton, Pure Appl. Chem. 61, 1395 (1989).
S. W. Benson, F. R. Cruickshank, D. M. Golden, et al., Chem. Rev. 69, 279 (1969).
S. W. Benson, Thermochemical Kinetics, 2nd ed (Wiley, New York, 1976).
N. Cohen and S. W. Benson, Chem. Rev. 93, 2419 (1993).
J. B. Pedley, R. D. Naylor, and S. P. Kirby, Thermochemical Data of Organic Compounds, Ed. by J. B. Pedley, 2nd ed. (Chapman and Hall, London, New York, 1986).
G. Ya. Kabo, G. N. Roganov, and Z. A. Filippenko, Zh. Fiz. Khim. 61, 2885 (1987).
I. V. Garist, S. V. Petrova-Kuminskaya, G. N. Roganov, et al., Zh. Fiz. Khim. 74, 397 (2000) [Russ. J. Phys. Chem. A 74, 327 (2000)].
G. F. Forsythe, M. A. Malcolm, and C. B. Moler, Computer Methods for Mathematical Computations, Prentice Hall Professional Technical Reference (Prentice Hall Englewood Cliffs, NJ, 1977).
A. M. Chibiryaev, A. Yermakova, and I. V. Kozhevnikov, J. Supercrit. Fluids 51, 295 (2010).
Handbook of Chemistry and Physics, Ed. by D. R. Lide, 81st ed. (CRC Press,, Raton Boca, FL, 2000).
Handbook of Data on Organic Compounds, Ed. by D. R. Lide and G. W. A. Milne, 3nd ed. (CRC Press, Boca Raton, FL, 1993).
Aldrich Catalog/Handbook of Fine Chemicals (Aldrich Chemical Company, Milwaukee, WI, 1990).
W. V. Steele, R. D. Chirico, A. B. Cowell, et al., J. Chem. Eng. Data 47, 667 (2002).
Experimental Data, www.reaxys.com.
I. I. Bardyshev and L. A. Popova, Rus. J. Org. Chem. 30, 1026 (1994).
C. M. Williams and D. Whittaker, J. Chem. Soc. B: Phys. Org., 668 (1971).
B. E. Poling, J. M. Prausnitz, and J. P. O’Connell, The Properties of Gases and Liquids, 5th ed. (McGraw-Hill, New York, 2004).
S. I. Sandler, Chemical, Biochemical, and Engineering Thermodynamics, 4th ed. (Wiley, New York, 2006).
G. Soave, Chem. Eng. Sci. 27, 1197 (1972).
D. W. Marquardt, J. Soc. Ind. App. Math. 11, 431 (1963).
L. A. Goldblatt and S. Palkin, J. Am. Chem. Soc. 63, 3517 (1941).
R. L. Burwell, Jr., J. Am. Chem. Soc. 73, 4461 (1951).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Zhurnal Fizicheskoi Khimii, 2011, Vol. 85, No. 8, pp. 1460–1471.
This article was translated by the authors.
Rights and permissions
About this article
Cite this article
Chibiryaev, A.M., Ermakova, A. & Kozhevnikov, I.V. Reaction reversibility in α-pinene thermal isomerization: improving the kinetic model. Russ. J. Phys. Chem. 85, 1347–1357 (2011). https://doi.org/10.1134/S0036024411080061
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024411080061