Abstract
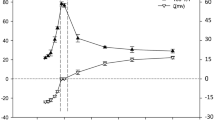
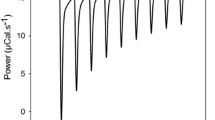
The effects of inorganic mono- and divalent salts of different types on how the cation polyelectrolyte polyallylamine hydrochloride (PAA) binds with the oligomer enzyme urease were studied. It was shown that in solutions of the monovalent salts NaCl, KCl, and NH4Cl, polyelectrolyte-protein complexes formed by electrostatic interactions, which decreased monotonically as the salt concentrations increased according to the classic law of statistical physics, correlating the Debye radius with the ionic strength of the solution. In solutions of the divalent salts Na2SO4 and (NH4)2SO4, the efficiency of the formation of the polyelectrolyte-protein complexes changed abruptly (the enzyme was drastically activated) at low salt concentrations (∼0.6–0.8 mM), which was not consistent with the classic theory of charge interactions in solutions with different ionic strengths. Turbidimetric titration at different salt concentrations in the given range revealed a high aggregative ability for sulfates and low ability for chlorides. It was concluded that the anomalies in the concentration dependence of the enzyme activity and aggregative ability were related to the formation of stable bonds PAA to the divalent SO 2−4 anion, which increased drastically when the ratio of anion concentration to the number of positively charged PAA monomers in solution reached 1: 2.
Similar content being viewed by others
References
M. Bernfield, M. Gotte, P. W. Park, et al., An. Rev. Biochem. 68, 729 (1999).
H. M. Himmel, M. Pietsch, U. Streller, et al., Basic Res. Cardiol. 97(6), 434 (2002).
E. Seyrek, P. L. Dubin, C. Tribet, and E. A. Gamble, Biomacromolecules 4, 273 (2003).
S. Mulrooney, T. Zakharian, R. A. Schaller, and R. P. Hausinger, Archiv. Biochem. Biophys. 394, 280–282 (2001).
S. D. Cesareo and S. R. Langton, FEMS Microbiol. Lett. 99, 15 (1992).
M. B. Moncrief, L. G. Hom, E. Jabri, et al., Protein Sci. 4, 2234 (1995).
R. Bhowmick and M. V. Jagannadham, Protein J. 25, 399 (2006).
S. Mulrooney, Archiv. Biochem. Biophys. 394, 280 (2001).
M. A. Pearson, I. S. Park, R. A. Schaller, et al., Biochemistry 39, 8575 (2000).
R. P. Hausinger and P. A. Karplus, Handbook of Metalloproteins, Ed. by K. Wieghardt, R. Huber, T. L. Poulos, and A. Messerschmidt (Wiley, West Sussex, UK, 2001), pp. 867–879.
A. B. Kayitmazer, S. P. Strand, S. P. Tribet, et al., Biomacromolecules 8, 3568 (2007).
C. Follmer, Phytochemistry 69, 18 (2008).
K. Kaibara, T. Okazaki, H. B. Bohidar, and P. L. Dubin, Biomacromolecules 1, 100 (2000).
H. L. Mobley, M. D. Island, and R. P. Hausinger, Microbiol. Rev. 59, 451 (1995).
S. A. Tikhonenko, E. A. Saburova, Yu. N. Dybovskaya, and B. I. Sukhorukov, Zh. Fiz. Khim. 82, 554 (2008) [Russ. J. Phys. Chem. 82, 468 (2008)].
R. Feinman, R. Leiton, and M. Sands, Feinmann Lectures in Physics (Addison-Wesley, Reading, Mass, 1964; Mir, Moscow, 1966).
P. H. Von-Hippel and A. Hamabata, J. Mechanochem. Cell Motic. 2, 127 (1973).
R. L. Baldwin, Biophys. J. 71, 2056 (1996).
J. Musil, O. Novakova, and K. Kunz, Biochemistry in Schematic Perspective (Prague, 1977; Mir, Moscow, 1984).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.A. Tikhonenko, E.A. Saburova, E.N. Durdenko, B.I. Sukhorukov, 2009, published in Zhurnal Fizicheskoi Khimii, 2009, Vol. 83, No. 10, pp. 1966–1974.
Rights and permissions
About this article
Cite this article
Tikhonenko, S.A., Saburova, E.A., Durdenko, E.N. et al. Enzyme-polyelectrolyte complex: Salt effects on the reaction of urease with polyallylamine. Russ. J. Phys. Chem. 83, 1781–1788 (2009). https://doi.org/10.1134/S0036024409100276
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024409100276