Abstract
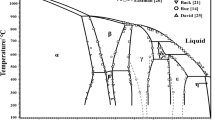
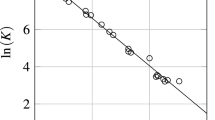
The isotropic liquid-nematic phase transition and the kinetics of growth of nematic phase drops during this phase transition were studied by polarization optical microscopy and IR spectroscopy for a four-component system during cooling. The statistical drop size distribution was described in terms of the equilibrium thermodynamics of irreversible processes. An analysis of the time dependences of the mean diameter of drops showed the presence of two kinetic stages of nematic phase growth and allowed them to be described by the universal law of cluster growth. In conformity with the Gibbs phase rule, nematic phases with different compositions can coexist at equilibrium.
Similar content being viewed by others
References
Handbook of Liquid Crystals, Ed. by D. Demus, J. Goodby, G. W. Gray, H.-W. Spiess, and V. Vill (Wiley-VCH, Weinheim, 1998).
A. Münster, Chemical Thermodynamics (Verlag Chemie, Weinheim, 1969; Mir, Moscow, 1971).
G. W. Gray and J. W. Goodby, Smectic Liquid Crystals-Textures and Structure (Wiley, Glasgow, 1984).
K. Diekmann, M. Schumacher, and H. Stegemeyer, Liq. Cryst. 25, 349 (1998).
H. G. Kilian, R. Metzler, and B. Zink, J. Chem. Phys. 107, 8697 (1997).
H. G. Kilian, S. Bronnikov, and T. Sukhanova, J. Phys. Chem. B 107, 13575 (2003).
A. G. Nasonov, S. V. Bronnikov, and I. Dirking, Zh. Fiz. Khim. 80, 427 (2006) [Russ. J. Phys. Chem. 80, 348 (2006)].
R. B. Blumstein, E. M. Stickles, and A. Blumstein, Mol. Cryst. Liq. Cryst. 82, 205 (1982).
A. J. Bray, Adv. Phys. 51, 481 (2002).
I. Dierking, J. Phys. Chem. B 104, 10642 (2000).
A. G. Nasonov, S. V. Bronnikov, and K. Raklesh, Zh. Fiz. Khim. 82, 651 (2008) [Russ. J. Phys. Chem. 82, 554 (2008)].
A. G. Nasonov, V. Kozan, and S. V. Bronnikov, Zh. Fiz. Khim. 82, 2025 (2008) [Russ. J. Phys. Chem. 82, 1814 (2008)].
B. A. H. Huisman and A. Fasolino, Phys. Rev. E 76, 021706 (2007).
G. Madras and B. J. McCoy, J. Chem. Phys. 117, 8042 (2002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.V. Kostromin, S.V. Bronnikov, V.V. Zuev, 2009, published in Zhurnal Fizicheskoi Khimii, 2009, Vol. 83, No. 10, pp. 1872–1876.
Rights and permissions
About this article
Cite this article
Kostromin, S.V., Bronnikov, S.V. & Zuev, V.V. Heterogeneous equilibria and the kinetics of isotropic liquid-nematic phase transition in a multicomponent mixture 4-alkyl-4′-cyanobiphenyls. Russ. J. Phys. Chem. 83, 1689–1693 (2009). https://doi.org/10.1134/S0036024409100100
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024409100100