Abstract
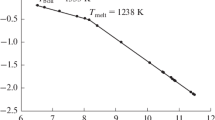
The vaporization of praseodymium triiodide was studied by high-temperature mass spectrometry. Monomeric (PrI3) and dimeric (Pr2I6) molecules and the PrI −4 and Pr2I −7 negative ions were recorded in saturated vapor over the temperature range 842–1048 K. The partial pressures of neutral vapor components were determined. The enthalpies of sublimation Δs H o(298.15 K) in the form of monomers (291 ± 10 kJ/mol) and dimers (400 ± 30 kJ/mol) were calculated by the second and third laws of thermodynamics. The equilibrium constants of ion-molecular reactions were measured and the enthalpies of the reactions determined. The enthalpies of formation Δf H o(298.15 K) of molecules and ions in the gas phase were calculated (−373 ± 11, −929 ± 31, −865 ± 25, and −1433 ± 48 kJ/mol for PrI3, Pr2I6, PrI −4 , and Pr2I −7 , respectively).
Similar content being viewed by others
References
J. F. Waymouth, Electric Discharge Lamps (M.I.T. Press, Cambridge, MA, 1971), p. 206.
D. E. Work, Lighting Res. Technol. 13, 143 (1981).
K. Hilpert and U. Niemann, Thermochim. Acta 299, 49 (1997).
T. Markus, U. Niemann, and K. Hilpert, J. Phys. Chem. Solids 66, 372 (2005).
V. E. Shimazaki and K. Niwa, Z. Anorg. Allg. Chem. 314, 21 (1962).
C. Hirayama and F. E. Camp, J. Chem. Eng. Data 17, 415 (1972).
A. R. Villani, B. Brunetti, and V. Piacente, J. Chem. Eng. Data 45, 1167 (2000).
C. Hirayama and P. M. Castle, J. Phys. Chem. 77, 3110 (1973).
K. Hilpert and K. Ruthardt, Ber. Bunsen-Ges. Phys. Chem. 91, 724 (1987).
A. M. Pogrebnoi, L. S. Kudin, A. Yu. Kuznetsov, and M. F. Butman, Rapid Commun. Mass Spectrom. 11,1536 (1997).
Thermodynamic Properties of Pure Substances: A Handbook, Ed. by V. P. Glushko (Nauka, Moscow, 1978) [in Russian].
Electronic Database IVTANTHERMO-2007 (private communication).
E. L. Osina, V. S. Yungman, and L. N. Gorokhov, Electronic Journal “Issledovano v Rossii” 8, 124 (2000) (http://zhurnal.ape.relarn.ru/artisles/2000/008.pdf).
A. Kovács, Chem. Phys. Lett. 329, 238 (2000).
W. E. Martin, R. Zalubas, and L. Hagan, Atomic Energy Levels: The Rare-Earth Elements, NSRDS NBS60 (National Bureau of Standards, Washington, DC, 1978).
P. W. Gilles, B. R. Conard, R. I. Sheldon, and J. E. Bennet, in Thermodynamics of Nuclear Materials (IAEA, Vienna, 1975), Vol. 2, p. 499.
D. E. Vorob’ev, Extended Abstract of Candidate’s Dissertation in Chemistry (Ivanovo State Univ. of Chemical Technology, Ivanovo, 2005).
V. G. Solomonic, A. N. Smirnov, and M. A. Mileev, Russ. J. Coord. Chem. 31, 203 (2005).
E. H. P. Cordfunke and R. J. M. Konings, Thermochim. Acta 375, 17 (2001).
A. M. Pogrebnoi, Extended Abstract of Doctoral Dissertation in Chemistry (Inst. of the Solution Chemistry, Russ. Acad. Sci., Ivanovo, 2004).
A. M. Sapegin, A. V. Baluev, and O. P. Charkin, Russ. J. Inorg. Chem. 32, 318 (1987).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.B. Motalov, L.S. Kudin, T. Markus, 2009, published in Zhurnal Fizicheskoi Khimii, 2009, Vol. 83, No. 3, pp. 418–425.
Rights and permissions
About this article
Cite this article
Motalov, V.B., Kudin, L.S. & Markus, T. The thermodynamic characteristics of vaporization of praseodymium triiodide. Russ. J. Phys. Chem. 83, 338–345 (2009). https://doi.org/10.1134/S0036024409030030
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024409030030