Abstract
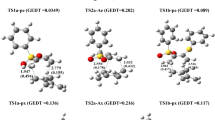
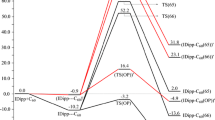
Density functional theory with the B3LYP hybrid functional and 6–31G* basis set was used to study the geometric and electronic structure of H2C = CHR (R = H, CH3, C2H5, C3H7, C4H9, and C5H11) olefins, their carbocations formed in the addition of the proton to the olefins, R′-S-H aliphatic thiols (R′ = H, CH3, C2H5, and C3H7), the products of the addition of thiols to carbocations, and the final products of the addition of thiols to olefins. The proton affinity of the olefins and the products of the addition of thiols to olefins was calculated. The conclusion was drawn that the limiting stage in the nonradical addition of thiols to olefins catalyzed by acids was proton transfer from the protonated reaction product to the olefin. The theoretical results were compared with the experimental data on the electrophilic addition of polymercaptan to heptene-1.
Similar content being viewed by others
References
E. N. Prilezhaeva and M. F. Shostakovskii, Usp. Khim. 32(8), 897 (1963).
I. V. Koval’, Usp. Khim. 62(8), 813 (1993).
A. D. Becke, J. Chem. Phys. 98(7), 5648 (1993).
T. H. Dunning, Jr., in Modern Theoretical Chemistry, Ed. by H. F. Schaefer III (Plenum, New York, 1976), pp. 1–28.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 98, Revision A.5 (Gaussian, Inc., Pittsburgh, PA, 1998).
L. V. Gurvich, G. V. Karachentsev, V. N. Kondrat’ev, et al., Dissociation Energies of Chemical Bonds, Ionization Potentials, and Electron Affinities (Nauka, Moscow, 1974) [in Russian].
J. E. Szulejko and T. B. Mc Mahon, J. Am. Chem. Soc. 115(17), 7839 (1993).
B. J. Smith and L. Radon, J. Am. Chem. Soc. 115(11), 4885 (1993).
Yu. A. Borisov, Zh. Strukt. Khim. 43(5), 803 (2002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © Yu.A. Borisov, A.K. Dyusengaliev, K.I. Dyusengaliev, T.P. Serikov, 2008, published in Zhurnal Fizicheskoi Khimii, 2008, Vol. 82, No. 12, pp. 2319–2324.
Rights and permissions
About this article
Cite this article
Borisov, Y.A., Dyusengaliev, A.K., Dyusengaliev, K.I. et al. The electrophilic addition of thiols to olefins: A theoretical and experimental study. Russ. J. Phys. Chem. 82, 2092–2096 (2008). https://doi.org/10.1134/S0036024408120212
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024408120212