Abstract
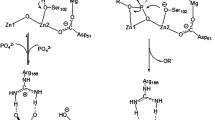
A comparative study was performed to examine the catalytic properties of alkaline phosphatases from bacteria Escherichia coli and bovine and chicken intestines. The activity of enzyme dimers and tetramers was determined. The activity of the dimer was three or four times higher than that of the tetramer. The maximum activity and affinity for 4-nitrophenylphosphate was observed for the bacterial alkaline phosphatase (K M = 1.7 × 10−5 M, V max = 1800 μmol/(min mg of protein) for dimers and V max = 420 μmol/(min mg of protein) for tetramers). The Michaelis constants were equal for two animal phosphatases in various buffer media (pH 8.5) ((3.5 ± 0.2) × 10−4 M). Five buffer systems were investigated: tris, carbonate, hepes, borate, and glycine buffers, and the lowest catalytic activity of alkaline phosphatases at equal pH was observed in the borate buffer (for enzyme from bovine intestine, V max = 80 μmol/(min mg of protein)). Cu2+ cations formed a complex with tris-(oxymethyl)-aminomethane (tris-HCl buffer) and inhibited the intestine alkaline phosphatases by a noncompetitive mechanism.
Similar content being viewed by others
References
R. B. McComb, G. N. Bowers, and S. Posen, Alkaline Phosphatases (Plenum, New York, 1979).
E. E. Kim and H. W. Wyckoff, Clin. Chim. Acta 186, 175 (1990).
M. H. le Du, T. Stigbrand, M. J. Taussig, et al., J. Biol. Chem. 276, 9158 (2001).
M. de Backer, S. McSweeney, H. B. Rasmussen, et al., J. Mol. Biol. 318, 1265 (2002).
E. Wang, D. Koutsioulis, H.-K. S. Leiros, et al., J. Mol. Biol. 366, 1318 (2007).
R. A. Thomas and J. F. Kirsch, Biochemistry 23, 5328 (1980).
G. F. Verpooten, M. F. Hoylaerts, E. J. Nouwen, and M. E. de Broe, Clin. Chim. Acta 186, 225 (1990).
E. Sarciron and A.-F. Petavy, J. Parasitology 80, 667 (1994).
S. Zarra, J. Boudrant, and E. R. Kantrowitz, J. Inorg. Biochem. 98, 575 (2004).
S. Organovic and M. Pavela-Vrancic, Eur. J. Biochem. 270, 4356 (2003).
M. Bortolato, F. Besson, and B. Roux, Proteins: Struct., Funct., Genet. 37, 310 (1999).
Q.-X. Chen, W.-Z. Zheng, J.-Y. Lin, et al., Int. J. Biochem. Cell Biol. 32, 879 (2000).
J. Wang, K. A. Steiglitz, and E. R. Kantrowitz, Biochemistry 44, 8378 (2005).
B. I. Kurganov, Allosteric Enzymes (Nauka, Moscow, 1978) [in Russian].
C. J. Martin, J. Inorg. Biochem. 58, 89 (1995).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.F. Atyaksheva, E.S. Chukhrai, O.M. Poltorak, 2008, published in Zhurnal Fizicheskoi Khimii, 2008, Vol. 82, No. 11, pp. 2164–2169.
Rights and permissions
About this article
Cite this article
Atyaksheva, L.F., Chukhrai, E.S. & Poltorak, O.M. The catalytic properties of alkaline phosphatases under various conditions. Russ. J. Phys. Chem. 82, 1947–1951 (2008). https://doi.org/10.1134/S0036024408110265
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024408110265