Abstract
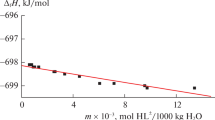
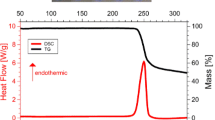
The energy of combustion of N-(carboxymethyl)aspartic acid (CMAA) was determined by bomb calorimetry in oxygen. The standard enthalpies of combustion and formation of crystalline N-(carboxymethyl)aspartic acid were calculated. The heat effects of solution of crystalline CMAA in water and a solution of sodium hydroxide were measured at 298.15 K by direct calorimetry. The standard enthalpies of formation of CMAA and its dissociation products in aqueous solution were determined.
Similar content being viewed by others
References
S. V. Sharov, V. M. Nikol’skii, and I. P. Gorelov, Zh. Neorg. Khim. 50(6), 1047 (2005) [Russ. J. Inorg. Chem. 50 (6), 966 (2005)].
V. P. Vasil’ev and A. V. Volkov, Zh. Fiz. Khim. 70(5), 931 (1996) [Russ. J. Phys. Chem. 70 (5), 866 (1996)].
F. D. Rossini, in Experimental Thermochemistry, Ed. by F. D. Rossini (Wiley, New York, 1956).
V. P. Vasil’ev, E. V. Kozlovskii, and S. F. Ledenkov, Zh. Fiz. Khim. 61(5), 1429 (1987).
P. P. Korostelev, Preparation of Solutions for Chemical Analysis (Akad. Nauk SSSR, Moscow, 1962), p. 398 [in Russian].
V. P. Vasil’ev, V. A. Borodin, and E. V. Kozlovskii, Application of Computers in Chemical Analysis Calculations (Vysshaya Shkola, Moscow, 1993), p. 81 [in Russian].
Thermal Constants of Substances: A Handbook, Ed. by V. P. Glushko et al. (VINITI, Moscow, 1965–1971), Vol. 3 [in Russian].
Yu. I. Aleksandrov, V. N. Oleinik, and T. R. Usvyatseva, in Proceedings of USSR Metrological Institutes (Izd. Standartov, Moscow, 1971), No. 29 (189), p. 155 [in Russian].
V. P. Vasil’ev, V. A. Borodin, and S. B. Kopnyshev, Zh. Fiz. Khim. 65(1), 55 (1991).
L. A. Kochergina, O. N. Krutova, and A. V. Volkov, Zh. Fiz. Khim. 79(12), 2206 (2005) [Russ. J. Phys. Chem. 79 (12), 1967 (2005)].
I. A. Rumyantseva, Candidate’s Dissertation in Chemistry (Ivanovo State Univ. of Chemical Technology, Ivanovo, 2006).
C. M. Davies, J. Chem. Soc., p. 2093 (1983).
Author information
Authors and Affiliations
Additional information
Original Russian Text © A.I. Lytkin, N. V. Chernyavskaya, A.V. Volkov, V.M. Nikol’skii, 2007, published in Zhurnal Fizicheskoi Khimii, 2007, Vol. 81, No. 7, pp. 1175–1179.
Rights and permissions
About this article
Cite this article
Lytkin, A.I., Chernyavskaya, N.V., Volkov, A.V. et al. The standard enthalpies of formation of crystalline N-(carboxymethyl)aspartic acid and its aqueous solutions. Russ. J. Phys. Chem. 81, 1025–1029 (2007). https://doi.org/10.1134/S0036024407070035
Received:
Issue Date:
DOI: https://doi.org/10.1134/S0036024407070035