Abstract
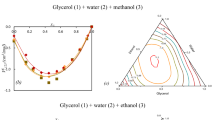
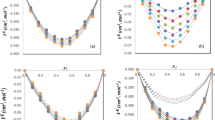
Based on the assumption that there exists a distribution of hydrates over the hydration number, expressions for the hydration number, activity coefficients of the components, and excess Gibbs energy were obtained. It was demonstrated that the van Laar formula for the activity coefficients corresponds to the Poisson distribution of hydrates. It was established that, at a constant ratio of the variance to the mathematical expectation of the distribution of hydrate, the model’s equations adequately describe the available experimental data on the vapor pressure and water activity for the glycerol-water system over the entire concentration range.
Similar content being viewed by others
References
G. Scatchard, W. J. Hamer, and S. E. Wood, J. Am. Chem. Soc. 60(12), 3061 (1938).
E. C. H. To, J. V. Davies, M. Ticker, et al., J. Solution Chem. 28(10), 1137 (1999).
C. Marcolli and Th. Peter, Atmos. Chem. Phys. Discuss. 5, 1501 (2005).
Y. Marcus, Phys. Chem. Chem. Phys. 2, 4891 (2000).
G. Scatchard, J. Am. Chem. Soc. 43, 2387 (1921).
G. Scatchard, J. Am. Chem. Soc. 43, 2406 (1921).
A. Callendar, Proc. R. Soc. (London), Ser. A 80, 466 (1908).
R. A. Robinson and R. H. Stokes, Electrolyte Solutions, 2nd ed. (Butterworth, London, 1965).
R. A. Robinson and R. H. Stokes, J. Phys. Chem. 65, 1954 (1961).
R. A. Robinson and R. H. Stokes, J. Phys. Chem. 70, 2126 (1966).
G. R. Allakhverdov, Zh. Fiz. Khim. 66, 1802 (1992).
H. Z. Schonert, Z. Phys. Chem. (Leipzig) 150, 163 (1968).
H. Z. Schonert, Z. Phys. Chem. (Leipzig) 150, 181 (1968).
A. M. Rudakov and V. V. Sergievskii, Inzh. Fiz., No. 1, 31 (2001).
A. M. Rudakov, E. O. Khomchenko, and V. V. Sergievskii, Inzh. Fiz., No. 3, 9 (2003).
A. M. Rudakov and V. V. Sergievskii, Zh. Fiz. Khim. 71, 1420 (1997) [Russ. J. Phys. Chem. 71, 1272 (1997)].
A. M. Rudakov, E. Yu. Zhavoronkov, and V. V. Sergievskii, Zh. Fiz. Khim. 71, 1628 (1997) [Russ.J. Phys. Chem. 71, 1464 (1997)].
I. Prigogine and R. Defay, Chemical Thermodynamics (Longman, London, 1967).
Author information
Authors and Affiliations
Additional information
Original Russian Text © A.M. Rudakov, V.V. Sergievskii, 2006, published in Zhurnal Fizicheskoi Khimii, 2006, Vol. 80, No. 11, pp. 2026–2031.
Rights and permissions
About this article
Cite this article
Rudakov, A.M., Sergievskii, V.V. Activities of the components of glycerol-water binary solutions at 298.15 K. Russ. J. Phys. Chem. 80, 1804–1808 (2006). https://doi.org/10.1134/S0036024406110227
Issue Date:
DOI: https://doi.org/10.1134/S0036024406110227