Abstract
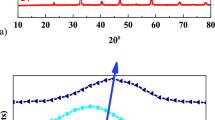
Manganites doped with potassium ions La1 – xKxMnO3, where х = 0.0, 0.1, and 0.15, have been obtained by extraction-pyrolytic method at low temperature. The compounds have been studied by X-ray powder diffraction, electronic paramagnetic and ferromagnetic resonances. Unit cell parameters of La1 – xKxMnO3 samples have been calculated. According to magnetic resonance spectroscopy, La1 – xKxMnO3 exhibits phase delamination into paramagnetic and ferromagnetic phases. The fraction of the latter phase increases when temperature decreases. The temperature of transition from paramagnetic into ferromagnetic phase (Curie temperature, TC) for La1 – xKxMnO3 is –17.4, –13.7, and –4.8°С at х = 0.0, 0.1, and 0.15, respectively. The reason of symbate change in TC and potassium ion concentration in La1 – xKxMnO3 is supposed to be the change in the content of ferromagnetic pairs Mn3+–О2––Mn4+ in the manganites.




Similar content being viewed by others
REFERENCES
X. Guan, H. Li, Sh. Jin, et al., Ceram. Int. 47, 18931 (2021). https://doi.org/10.1016/j.ceramint.2021.03.235
N. D. Sharma, S. Sharma, N. Choudhary, et al., Ceram. Int. 45, 13637 (2019). https://doi.org/10.1016/j.ceramint.2019.04.004
N. Hur, S. Park, P. A. Shama, et al., Nature 429, 392 (2004).
C. Shivakumara and M. B. Bellakki, Bull. Mater. Sci. 32, 443 (2009).
M. W. Shaikh and D. Varshney, Mater. Chem. Phys. 134, 886 (2012).
L. E. Gonchar, Phys. Solid State 61, 728 (2019). https://doi.org/10.1134/S1063783419050093
D. I. Pchelina, V. D. Sedykh, N. I. Chistyakova, et al., J. Phys. Chem. Solids 159, 110268 (2021). https://doi.org/10.26201/ISSP.2020/FKS-2.330
V. Markovich, G. Jung, I. Fita, et al., J. Phys. D: Appl. Phys. 41, 185001 (2008). https://doi.org/10.1088/0022-3727/41/18/185001
C. Zener, Phys. Rev. 82, 403 (1951). https://doi.org/10.1103/PhysRev.82.403
J. M. D. Coey, M. Viret, and S. Molnár, Adv. Phys. 48, 167 (1999). https://doi.org/10.1080/000187399243455
R. B. Griffiths, Phys. Rev. Lett. 23, 17 (1969). https://doi.org/10.1103/PhysRevLett.23.17
Y. Ying, T. W. Eom, N. V. Dai, et al., J. Magn. Magn. Mater. 323, 94 (2011). https://doi.org/10.1016/j.jmmm.2010.08.036
J. Deisenhofer, D. Braak, and K. H.-A. von Nidda, et al., Phys. Rev. Lett. 95, 257202 (2005). https://doi.org/10.1103/PhysRevLett.95.257202
R. M. Eremina, J. V. Yatsyk, Ya. M. Mukovskii, et al., JETP Lett. 85, 51 (2007). https://doi.org/10.1134/S0021364007010109
F. N. Bukhan’ko and A. F. Bukhan’ko, Phys. Solid State 64, 802 (2022). https://doi.org/10.21883/PSS.2022.07.54584.304
G. S. Patrin, Y. G. Shiyan, S. A. Yarikov, et al., Phys. Solid State 62, 1350 (2020). https://doi.org/10.1134/S1063783420080272
Yu. M. Nikolaenko, N. B. Efros, D. O. Fedyuk, et al., Phys. Solid State 64, 798 (2022). https://doi.org/10.21883/PSS.2022.07.54583.236
A. A. Povzner, A. G. Volkov, E. I. Lopatko, et al., Phys. Solid State 65, 531 (2023). https://doi.org/10.21883/PSS.2023.04.55991.528
A. V. Fedorova, N. V. Chezhina, E. A. Ponomareva, et al., Russ. J. Gen. Chem. 93, 64 (2023). https://doi.org/10.31857/S0044460X23010158
A. V. Fedorova, N. V. Chezhina, and V. V. Shilovskikh, Russ. J. Gen. Chem. 93, 68 (2023). https://doi.org/10.1134/S1070363223010103
Ya. Mittova, N. S. Perov, E. V. Tomina, et al., Inorg. Mater. 57, 1340 (2021). https://doi.org/10.1134/S0020168521130033
N. I. Steblevskaya, M. A. Medkov, and S. B. Yarusova, Preparation and Properties of Functional Materials Based on Rare Earth and Rare Metal Oxides (VGUES, Vladivostok, 2021).
N. I. Steblevskaya, M. V. Belobeletskaya, I. A. Tka-chenko, et al., Russ. J. Inorg. Chem. 61, 880 (2016). https://doi.org/10.1134/S0036023616070196
N. I. Steblevskaya, Theor. Found. Chem. Eng. 56, 905 (2022). https://doi.org/10.1134/S0040579522050165
I. K. Kamilov, A. G. Gamzatov, A. B. Batdalov, et al., Phys. Solid State 52, 735 (2010).
Yu. G. Chukalkin and A. E. Teplykh, Phys. Solid State 48, 2310 (2006).
Yu. A. Izyumov and Yu. N. Skryabin, Usp. Fiz. Nauk 172, 121 (2001).
B. Dabrovski, K. Rogacki, X. Xiong, et al., Phys. Rev. 70, 5716 (1988).
V. P. S. Awana, E. Schimit, E. Gmelin, et al., J. Appl. Phys. 87, 5034 (2000).
N. Guskos, G. Zolnierkiewicz, A. Guskos, et al., Nanotechnology in the Security Systems. NATO Science for Peace and Security Series C: Environmental Security (Springer, Dordrecht, 2015). https://doi.org/10.1007/978-94-017-9005-5_4
V. Castel, J. B. Youssef, and C. Brosseau, J. Nanomater. 2007, 27437 (2007). https://doi.org/10.1155/2007/27437
S. A. Bouzid, A. C. Galca, M. Sajieddine, et al., J. Alloys Compd. 839, 155546 (2020).
S. Das and T. K. Dey, J. Alloys Compd. 440, 30 (2007). https://doi.org/10.1016/j.jallcom.2006.09.051
Funding
This work was performed under the State Assignments for the Institute of Chemistry, Far Eastern Branch, Russian Academy of Sciences (projects nos. FWFN (0205)-2022-0001 and FWFN (0205)-2022-0003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Additional information
Translated by I. Kudryavtsev
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Steblevskaya, N.I., Ziatdinov, A.M., Belobeletskaya, M.V. et al. Synthesis and Magnetic Resonance of Lanthanum Manganites Doped with Potassium Ions. Russ. J. Inorg. Chem. 68, 1737–1743 (2023). https://doi.org/10.1134/S0036023623602210
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023623602210