Abstract—
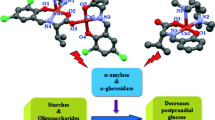
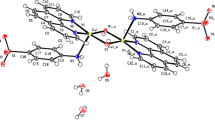
A series of four new copper(II) complexes [Cu2(2-BrC6H4CH2COO)4(Phen)2] (1), [Cu(2-BrC6H4CH2COO)2(Bipy)] (2), [Cu2(2-BrC6H4CH2COO)4(3-BrPy)2] (3) and [Cu2(2-BrC6H4CH2COO)4(3-MePy)2] (4) where Phen = 1,10-phenenthroline, Bipy = 2,2′-bipyridine, BrPy = bromopyridine and MePy = methylpyridine have been successfully synthesized and analyzed by elemental analysis, FT-IR, UV-Visible, and single crystal XRD. Complexes 1, 3 and 4 are dimeric and crystallize in the monoclinic space groups p21/c and C2/c, respectively. Distorted square pyramidal geometry around each Cu(II) ion in 1 is formed by the two nitrogen atoms from phenenthroline lying equatorially and three oxygen atoms from three monodentately coordinated carboxylate ligands. Two out of three ligands bridged the two metal ions with Cu···Cu intra dimer distance of 3.62 Å. In 3 and 4, four bidentate carboxylate ligands coordinate to two metal atoms which bridge them equatorially to give rise paddlewheel conformation with square pyramidal geometry around each metal atom. The axial positions of a square pyramid and trigonal bipyramid are occupied by nitrogen donor substituted pyridines with Cu···Cu intra dimer distances of 2.66 and 2.69 Å, respectively. The complex 2 being monomeric, crystallizes in the monoclinic space group C2/c with distorted square planar geometry around metal atom. A wide range of hydrogen bonding and π‒π stacking interactions are present throughout the crystal lattice in all complexes. DNA binding study through spectroscopic technique suggested strong capacity of complexes 1–4 to bind with DNA strand preferably through intercalative and groove binding modes with Kb values 6.03 × 103, 1.34 × 104, 3.18 × 104 and 3.14 × 104 M–1 respectively. The α-glucosidase and anticholinesterase enzyme inhibition assays were performed to investigate the potential of 1–4 as anti-diabetic and anti-alzheimer agents. The results revealed anti-diabetic nature of synthesized complexes with IC50 values in following order 1 < 2 < 3 < 4 with acarbose as control in concentration dependent manner. All these complexes exhibited mild activities against anticholinesterases with galantamine hydrobromide as control. The manuscript reports structurally diverse, bio-active complexes.







Similar content being viewed by others
REFERENCES
P. Jaividhya, M. Ganeshpandian, R. Dhivya, et al., Dalton Trans. 44, 11 997 (2015).
C. Rajarajeswari, M. Ganeshpandian, M. Palaniandavar, et al., J. Inorg. Biochem. 140, 255 (2014).
W. H. Mahmoud, G. G. Mohamed, and M. M. I. El-Dessouky, Int. J. Electrochem. Sci. 9, 1415 (2014).
A. J. Hallett, T. M. O’Brien, E. Carter, et al., Inorg. Chim. Acta 441, 86 (2016).
S. Chandralekaa, K. Ramyab, G. Chandramohana, et al., J. Saudi Chem. Soc. 18, 953 (2014).
G. S. Kurdekar, M. P. Sathisha, V. K. Revankar, et al., Eur. J. Med. Chem. 45, 455 (2010).
J. Sun and H. Xu, Molecules 15, 8349 (2010).
J. Boonmak, S. Youngme, T. Chotkhun, and J. Reedijk, Inorg. Chem. Commun. 11, 1231 (2008).
M. Devereux, M. McCann, V. Leon, et al., Met. Based Drugs 7, 275 (2000).
B. H. Ye, X. Y. Li, I. D. Williams, and X. M. Chen, Inorg. Chem. 41, 6426 (2002).
M. E. Russell, C. S. Hawes, A. Ferguson, et al., Dalton Trans. 42, 13 576 (2013).
C. D. Stewart, M. Pedraza, H. Arman, et al., J. Inorg. Biochem. 149, 25 (2015).
S. Gupta and M. K. Maheshwari, Chem. Sci. Trans. 3, 927 (2013).
P. M. Reddy, R. Rohini, E. R. Krishna, and A. Hu, V. Ravinder, Int. J. Mol. Sci. 13, 4982 (2012).
N. Nordin, W. Z. Samad, M. R. Yusop, and M. R. Othman, Malays. J. Anal. Sci. 19, 236 (2015).
Q. Wu, N. Xing, X. Liu, et al., Polyhedron 87, 390 (2015).
Z. N. Kadhim, J. Mater. Environ. Sci. 6, 693 (2015).
R. Alam, T. Mistri, P. Mondal, et al., Dalton Trans. 43, 2566 (2014).
N. A. Bailey, D. E. Fenton, R. Moody, et al., Dalton Trans. 11, 2519 (1987).
T. I. Kashar and A. H. El-Sehli, Eur. Sci. J. 12, 1857 (2016).
A. K. Jassal, S. Sharma, G. Hundal, and M. S. Hundal, Cryst. Growth Des. 15, 79 (2015).
G. L. Ellman, K. D. Courtney, V. Andres, and R. M. Featherstone, Biochem. Pharm. 7, 88 (1961).
Ferheen, S. Aziz-Ur-Rehman, N. Afza, et al., J. Enzyme Inhib. Med. Chem. 24, 1128 (2009).
Gorun, V. I. Proinov, V. Baltescu, et al., Anal. Biochem. 86, 324 (1978).
M. E. Owen, E. Carter, G. J. Hutchings, et al., Dalton Trans. 41, 11 085 (2012).
K. N. Lazarou, V. Psycharis, S. P. Perlepes, and C. P. Raptopoulou, Polyhedron 28, 1085 (2009).
S. Dey, S. Sarkar, H. Paul, et al., Polyhedron 29, 1583 (2010).
A. Colette, A. M. Ondoh, D. M. Yufanyi, and D. S. Y. Gaelle, Int. J. Chem. 7, 10 (2015).
T. Suksrichavalit, S. Prachayasittikul, T. Piacham, et al., Molecules 13, 3040 (2008).
D. L. Reger, J. J. Horger, A. Debreczeni, and M. D. Smith, Inorg. Chem. 50, 10 225 (2011).
L. F. Marquesa, M. V. Marinhoa, C. C. Correaa, et al., Inorg. Chim. Acta 368, 242 (2011).
B. Chen, F. R. Fronczek, B. H. Courtney, and F. Zapata, Cryst. Growth Des. 6, 825 (2006).
S. J. Jennifer and P. T. Muthiah, Chem. Cent. J. 42, 1 (2014).
P. M. Selvakumara, S. Nadellaa, J. Sahooa, et al., J. Coord. Chem. 66, 287 (2013).
C. S. Liu, J. J. Wang, L. F. Yan, et al., Inorg. Chem. 46, 6299 (2007).
M. Devereux, D. O’Shea, M. O’Connor, et al., Polyhedron 26, 4073 (2007).
A. N. Wein, R. Cordeiro, N. Owens, et al., J. Fluorine Chem. 130, 197 (2009).
V. Paredes-Garcia, R. C. Santana, R. Madrid, et al., Inorg. Chem. 15, 8369 (2013).
M. Iqbal, I. Ahmad, S. Ali, and M. Sohail, Polyhedron 50, 524 (2012).
Z. D. Matovic, V. D. Miletic, G. Samardzic, et al., Inorg. Chim. Acta 358, 3135 (2005).
P. J. K. Inba, B. Annaraj, S. Thalamuthu, and M. A. Neelakantan, Bioinorg. Chem. App. 2013, 1 (2013).
J. P. Barbier, A. El Biyyadh, C. Kappenstein, et al., Inorg. Chem. 24, 3615 (1985).
L. Zhang, L. Liu, G. F. Liu, et al., J. Chem. Crystallogr. 35, 583 (2005).
U. Mukhopadhyay, D. Choquesillo-Lazarte, J. Niclos-Gutierrez, and I. Bernal, Cryst. Eng. Commun. 6, 627 (2004).
J. C. Garcia-Ramos, A. Tovar-Tovar, J. Hernandez-Lima, et al., Polyhedron 30, 2697 (2011).
C. Hou, J. M. Shi, Y. M. Sun, et al., Dalton Trans. 43, 5970 (2008).
K. Miyamura, A, Mihara, T. Fujii, et al., J. Am. Chem. Soc. 117, 2377 (1995).
J. Sun, X. Tong, and H. Xu, Inorg. Chem. Commun. 13, 645 (2010).
E. N. M. Yusof, T. S. A. Ravoof, E. R. T. Tiekink, et al., J. Mol. Sci. 16, 11034 (2015).
D. S. Leela, B. Ushaiah, G. Anupama, et al., J. Fluoresc. 25, 185 (2015).
K. Khorsandi, R. Hosseinzadeh, and M. Gheshlagi, J. Appl. Sol. Chem. Model. 2, 105 (2013).
N. Shahabadi and S, Mohammadi, Bioinorg. Chem. App. 2012, 1 (2012).
M. Chikira, C. Hee Ng, and M. Palaniandavar, Int. J. Mol. Sci. 16, 22 754 (2015).
Funding
A.M. is thankful to Higher Education Commission of Pakistan for providing financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Afifa Mushtaq, Ali, S., Tahir, M.N. et al. Mixed-Ligand Cu(II) Carboxylates: Synthesis, Crystal Structure, FTIR, DNA Binding, Antidiabetic, and Anti-Alzheimer’s Studies. Russ. J. Inorg. Chem. 64, 1365–1378 (2019). https://doi.org/10.1134/S0036023619110147
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023619110147