Abstract
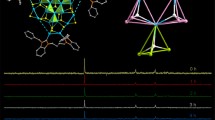
A new compound with the formula PbUO2(mac)4, where mac is the methacrylate ion CH2C(CH3)COO−, has been synthesized and studied by X-ray diffraction and IR spectroscopy. The equatorial plane of a UO7 pentagonal bipyramid contains the oxygen atoms of four anions, three of which are coordinated to the uranyl ions monodentately, and one is coordinated bidentately, forming mononuclear complexes [UO2(mac)4]2−. Each lead ion is bound to eight oxygen atoms of five methacrylate ions of three such complexes. As a result, lead ions bond uranium-containing complexes into electroneutral chains [PbUO2(mac)4], which are the main structural units of crystals. The different roles of the four crystallographically nonequivalent methacrylate ions in the crystal structure determine the crystal-chemical formulas of the chains as A′AB01B11(B21)2, where A′ = Pb2+; A=UO 2+2 ; B01, B11, and B21 = mac. The chains are linked by the system of hydrogen bonds to form a framework. The effect of the nature of R2+ cations on the structure of methacrylate uranylates is considered.
Similar content being viewed by others
References
T. Loiseau, I. Mihalcea, N. Henry, and C. Volkringer, 266–267, (2014). https://doi.org/10.1016/j.ccr.2013.08.038.
A. V. Savchenkov, A. V. Vologzhanina, D. V. Pushkin, and L. B. Serezhkina, Eur. J. Inorg. Chem. 2018, 1869 (2018). doi https://doi.org/10.1002/ejic.201701318
L. B. Serezhkina, A. V. Vologzhanina, V. V. Klepov, and V. N. Serezhkin, Kristallografiya 56, 138 (2011).
V. V. Klepov, E. V. Peresypkina, L. B. Serezhkina, et al., Zh. Neorg. Khim. 57, 1426 (2012).
L. B. Serezhkina, M. S. Grigor’ev, N. A. Shimin, et al., Russ. J. Inorg. Chem. 60, 672 (2015). doi https://doi.org/10.1134/S0036023615060121
V. V. Klepov, A. V. Vologzhanina, E. V. Alekseev, et al., Cryst. Eng. Commun. 18, 1723 (2016). doi https://doi.org/10.1039/C5CE01957E
V. V. Klepov, L. B. Serezhkina, D. V. Pushkin, et al., Eur. J. Inorg. Chem. 2016, 118 (2016). doi https://doi.org/10.1002/ejic.201501035
V. V. Klepov, L. B. Serezhkina, A. V. Vologzhanina, et al., Inorg. Chem. Commun. 46, 5 (2014).
V. V. Klepov, L. B. Serezhkina, M. S. Grigoriev, et al., Polyhedron 133, 40 (2017).
L. B. Serezhkina, M. S. Grigor’ev, V. V. Klepov, et al., Kristallografiya, No. 2 (2019).
R. B. White and H. W. Melvill, J. Soc. Dyers Colour. 65, 703 (1949). doi https://doi.org/10.1111/j.1478-4408.1949.tb02548.x
T. L. Cremers, P. G. Eller, and E. M. Larson, Acta Crystallogr., Sect. C 42, 1684 (1986). doi https://doi.org/10.1107/S0108270186090947
SAINT-Plus. Version 7.68 (Bruker, Madison, Wisconsin, USA, 2007).
SADABS. Bruker, Madison, Wisconsin, USA, 2007).
G. M. Sheldrick, Acta Crystallogr., Sect. A 64, 112 (2008). doi https://doi.org/10.1107/S0108767307043930
G. M. Sheldrick, Acta Crystallogr., Sect. C 71, 3 (2015). doi https://doi.org/10.1107/S2053229614024218
V. N. Serezhkin, Yu. N. Mikhailov, and Yu. A. Buslaev, Russ. J. Inorg. Chem. 42, 1871 (1997).
V. N. Serezhkin, A. V. Vologzhanina, L. B. Serezhkina, et al., Acta Crystallogr., Sect. B 65, 45 (2009). doi https://doi.org/10.1107/S0108768108038846
V. N. Serezhkin, M. O. Karasev, and L. B. Serezhkina, Radiochem. 55, 137 (2013). doi https://doi.org/10.1134/S106636221302001X
D. V. Pushkin, A. V. Marukhnov, and V. N. Serezhkin, Russ. J. Inorg. Chem. 51, 99 (2006). doi https://doi.org/10.1134/S0036023606010165
B. K. Vainshtein, Contemporary Crystallography (Nauka, Moscow, 1979), Vol. 2.
V. N. Serezhkin and Yu. A. Buslaev, Zh. Neorg. Khim. 42, 1178 (1997).
A. V. Marukhnov, D. V. Pushkin, and V. N. Serezhkin, Russ. J. Coord. Chem. 34, 570 (2008). doi https://doi.org/10.1134/S1070328408080034
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part A: Theory and Applications in Inorganic Chemistry (Wiley, 2009).
B. Wu, W. Lu, and X. Zheng, J. Coord. Chem. 56, 65 (2003).
Y. Zhu, W. Lu, and F. Chen, Acta Crystallogr., Sect. E 60, m1459 (2004). doi https://doi.org/10.1107/S1600536804022779
Author information
Authors and Affiliations
Corresponding author
Additional information
Russian Text © L.B. Serezhkina, M.S. Grigor’ev, N.A. Shimin, V.N. Serezhkin, 2019, published in Zhurnal Neorganicheskoi Khimii, 2019, Vol. 64, No. 3, pp. 272–280.
Rights and permissions
About this article
Cite this article
Serezhkina, L.B., Grigor’ev, M.S., Shimin, N.A. et al. Synthesis and Structure of PbUO2(CH2C(CH3)COO)4. Russ. J. Inorg. Chem. 64, 342–350 (2019). https://doi.org/10.1134/S0036023619030173
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023619030173