Abstract
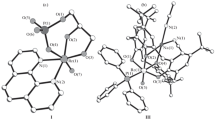
Structural features of 22 mononuclear octahedral monooxo d2-Re(V) complexes with tridentate chelating (О, N, О) ligands (Ltrin)—[ReO(Ltrin)(Lmono)2] and [ReO(Ltrin)(Lbim)] (Lmono is a monodentate ligand, is a bidentate ligand)—have been considered. Conditions for selecting ligands that can be located trans to the multiply bonded O(oxo) ligand are discussed.
Similar content being viewed by others
References
M. A. Porai-Koshits and E. A. Gilinskaya, Itogi Nauki Tekh. Ser. Kristallokhimiya, 126 (1966).
M. A. Porai-Koshits and L. O. Atovmyan, Koord. Khim. 1, 1271 (1975).
F. Griffith and C. Wicing, J. Chem. Soc. A 379 (1968).
M. A. Porai-Koshits, Izv. Yugosl. Kristallogr. Centra 9, 19 (1974)
M. A. Porai-Koshits and L. O. Atovmyan, The Crystal Chemistry of Molybdenum Coordination Compounds (Nauka, Moscow, 1974) [in Russan].
E. M. Shustorovich, M. A. Porai-Koshits, and Yu. A. Buslaev, Coord. Chem. Rev. 17, 1 (1975).
M. A. Porai-Koshits and V. S. Sergienko, Usp. Khim. 59, 86 (1990).
F. H. Allen, Acta Crystallogr., Sect. B 58, 380 (2002).
V. S. Sergienko and A. V. Churakov, Krystallografiya 59, 685 (2014).
V. S. Sergienko and A. V. Churakov, Krystallografiya 60, 365 (2014)
V. S. Sergienko, Zh. Neorg. Khim. 59, 457 (2014).
V. S. Sergienko, Zh. Neorg. Khim. 59, 1338 (2014).
V. S. Sergienko and A. V. Churakov, Russ. J. Inorg. Chem. 59, 1683 (2014).
V. S. Sergienko and A. V. Churakov, Russ. J. Inorg. Chem. 59, 1715 (2014).
V. S. Sergienko, Zh. Neorg. Khim. 60, 333 (2015).
V. S. Sergienko, Zh. Neorg. Khim. 60, 758 (2015).
V. S. Sergienko, Russ. J. Inorg. Chem. 61, 1461 (2016).
V. S. Sergienko, Zh. Neorg. Khim. 59, 957 (2014).
V. S. Sergienko, Russ. J. Inorg. Chem. 60, 1723 (2015).
V. S. Sergienko and A. V. Churakov, Russ. J. Inorg. Chem. 61, 873 (2016).
V. S. Sergienko, Zh. Neorg. Khim. 63, 601 (2018).
V. S. Sergienko, Zh. Neorg. Khim. 63, 7181 (2018).
V. S. Sergienko, Zh. Neorg. Khim. 59, 957 (2014).
V. S. Sergienko, Russ. J. Inorg. Chem. 60, 1723 (2015).
V. S. Sergienko and A. V. Churakov, Zh. Neorg. Khim. 61, 873 (2016).
V. S. Sergienko and A. V. Churakov, Krystallografiya 59, 341 (2014).
V. S. Sergienko and A. V. Churakov, Krystallografiya 58, 3 (2013).
P. Traar, A. Schröckeneder, M. E. Judmaier, et al. Eur. J. Inorg. Chem., 5718 (2010).
S. M. Harben, P. G. D. Smith, R. L. Beddoes, et al., J. Chem. Soc. Dalton Trans., 2777 (1997).
U. Mazzi, F. Refosco, G. Bandoli, and M. Nicolini, Transit. Met. Chem. 10, 121 (1985).
A. M. Kirillov, M. Haykka, M. V. Kirillova, and J. I. Pombiero, Adv. Synth. Catal. 347, 1435 (2005).
A. G. Orpen, I. Brommer, F. H. Allen, et al., J. Chem. Soc. Dalton Trans., 2897 (1999).
S. Savisch, N. Jáger, U. Schide, and E. Uhlemann, Struct. Chem. 10, 105 (1999).
C. Bolzati, M. Porchia, G. Bandoli, et al., Inorg. Chim. Acta 315, 205 (2001).
J. W. Babich, W. Graham, F. J. Femia, et al., Inorg. Chim. Acta 323, 23 (2001).
F. J. Femia, J. W. Babich, and J. Zubieta, Inorg. Chim. Acta 300–302, 462 (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary materials are available for this article at DOI: 10.1134/S0036023618140048 and are accessible for authorized users.
Electronic supplementary material
11502_2018_1698_MOESM1_ESM.pdf
Structural Features of Monomeric Octahedral Monooxo d2-Rhenium(V) Complexes [ReO(Ltrin)(Lmono)2] and [ReO(Ltrin)(Lbim)] with Oxygen Atoms of Tridentate Chelating (O, N, O) Ligands (Ltrin) (Review)
Rights and permissions
About this article
Cite this article
Sergienko, V.S. Structural Features of Monomeric Octahedral Monooxo d2-Rhenium(V) Complexes [ReO(Ltrin)(Lmono)2] and [ReO(Ltrin)(Lbim)] with Oxygen Atoms of Tridentate Chelating (O, N, O) Ligands (Ltrin) (Review). Russ. J. Inorg. Chem. 63, 1764–1771 (2018). https://doi.org/10.1134/S0036023618140048
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023618140048