Abstract
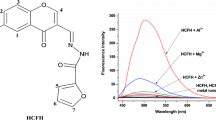
An easily available fluorescent sensor (L) based on salicylaldehyde has been investigated in this work. Chemosensor (L) was exhibited highly selective and sensitive fluorescence sensing ability for Fe3+ over other metal ions in H2O-DMF solution. The fluorescence quenching response of (L) for Fe3+ indcated that (L) can be used as “turn-off” fluorescent chemosensor to selectively detect Fe3+. The fluorescent sensor (L) was synthesized by the one pot condensation reaction of 2-[3-(2-formyl phenoxy)propoxy]benzaldehyde and 2-aminobenzenethiol in a 1 : 2 molar ratio and characterized by IR, NMR spectroscopy and elemental analysis.
Similar content being viewed by others
References
N. Karabocek, P. Ekmekcioglu, S. Muhsir, and S. Karabocek, Syn. React. Inorg. Met. 43, 768 (2013).
H. Komatsu, T. Miki, D. Citterio, et al., J. Am. Chem. Soc. 127, 10798 (2005).
Y. M. Yang, Q. Zhao, W. Feng, and F. Y. Li, Chem. Rev. 113, 192 (2013).
G. G. Hou, C. H. Wang, J. F. Sun, et al., Biochem. Biophys. Res. Co. 439, 459 (2013).
Chemosensors of Ion and Molecule Recognition, Ed. by J. P. Desvergne and A. W. Czarnik (Kluwer, Boston, 1997).
A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, et al., Chem. Rev. 97, 1515 (1997).
B. Valeur and I. Leray, Coord. Chem. Rev. 205, 3 (2000).
S. H. Kim, H. S. Choi, J. Kim, et al., Org. Lett. 12, 560 (2010).
Y. C. Hsieh, J. L. Chir, H. H. Wu, et al., Tetrahedron Lett. 51, 109 (2009).
Y. C. Hsieh, J. L. Chir, H. H. Wu, et al., Carbohydr. Res. 344, 2236 (2009).
H. N. Lee, H. N. Kim, K. M. K. Swamy, et al., Tetrahedron Lett. 49, 1261 (2008).
J. Kim, T. Morozumi, and H. Nakamura, Org. Lett. 9, 4419 (2007).
M. E. Huston, K. W. Haider, and A. W. Czarnik, J. Am. Chem. Soc. 110, 4460 (1988).
M. Formica, C. Fusi, L. Giorgi, and M. Micheloni, Coord. Chem. Rev. 256, 170 (2012).
T.-H. Ma, A.-J. Zhang, M. Dong, et al., J. Lumin. 130, 888 (2010).
M. Dong, Y.-W. Wang, and Y. Peng, Org. Lett. 12, 5310 (2010).
M. Dong, T.-H. Ma, A.-J. Zhang, et al., Dyes Pigm. 87, 164 (2010).
Y. Zhou, F. Wang, Y. Kim, et al., Org. Lett. 11, 4442 (2009).
V. Bhalla, Roopa, and M. Kumar, Org. Lett. 14, 2802 (2012).
S.-L. Hu, N.-F. She, G.-D. Yin, et al., Tetrahedron Lett. 48, 1591 (2007).
J. L. Bricks, A. Kovalchuk, C. Trieflinger, et al., J. Am. Chem. Soc. 127, 13522 (2005).
H. Ouchetto, M. Dias, R. Mornet, et al., Bioorg. Med. Chem. 13, 1799 (2005).
G. E. Tumambac, C. M. Rosencrance, and C. Wolf, Tetrahedron. 60, 11293 (2004).
Y. Ma, W. Luo, P.J. Quinn, et al., J. Med. Chem. 47, 6349 (2004).
R. Nudelman, O. Ardon, Y. Hadar, et al., J. Med. Chem. 41, 1671 (1998).
B. Mester, J. Libman, O. Dwir, et al., J. Am. Chem. Soc. 118, 12386 (1996).
I. Grabchev, J.-M. Chevelon, and X. Qian, New J. Chem. 27, 337 (2003).
J. J. R. Fausto da Silva and R. J. P. Williams, The Biological Chemistry of the Elements (Oxford University, New York, 1992).
Y. Xiang and A. Tong, Org. Lett. 8, 1549 (2006).
C. Brugnara, Clin. Chem. 49, 1573 (2003).
M. Zheng, H. Tan, Z. Xie, et al., Appl. Mater. Interfaces 5, 1078 (2013).
I. Yilmaz, H. Temeland, and H. Alp, Polyhedron 27, 152 (2008).
A. A. Ashraf, Tetrahedron 60, 1541 (2004).
W. Zoubi, F. Kandil, and M. K. Chebani, Spectrochim. Acta 69, 1909 (2011).
D. T. Quang, N. V. Hop, N. D. Luyen, et al., Luminescence 28, 222 (2012).
D. Schaming, C. Costa-Coquelard, I. Lampre, et al., Inorg. Chim. Acta 363, 2185 (2010).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Golbedaghi, R., Alavipour, E. & Shahsavari, M. Salicylaldehyde-Based ‘Turn-off’ Fluorescent Chemosensor with High Selectivity for Fe3+ in H2O-DMF Solution. Russ. J. Inorg. Chem. 63, 414–419 (2018). https://doi.org/10.1134/S0036023618030191
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023618030191