Abstract
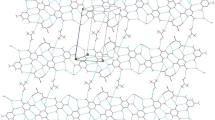
The reaction of the silver salts AgX (a: X = BF4-, b: X = ClO4-, c: X = OTf–) with α,α'-di(3/4-pyridylmethylene)cycloalkanones (L1–L3) and piperidones (L4–L7) results in the formation of coordination products of general composition [AgX(Ln)(solvent)] and [AgX(Ln)] (Ln = L1–L7). All complexes were characterized by elemental analysis and IR-spectroscopy. The structures of [Ag(ClO4)(L1)(MeC≡N)]∞ (1b · MeC≡N) and [Ag(ClO4)(L1)]∞ (1b) in the solid state are reported. In both structures {Ag(L1)}+ building units are linked to each other via Ag–Npyridine primary bonds resulting in the formation of infinite chains. In both structures the ligands L1 are fixed in transoid conformations, thus forming zig-zag polar chains. The structure of 1b · MeC≡N consists of pairs of tightly and loosely stacked chains. The tightly packed chains are weakly coupled by perchlorate anions acting as μ-bridges in between Ag(I) centers as well as by π–π-stacking interactions of unsaturated fragments of the respective ligands. In contrast, polar 2D layers composed of {Ag(L1)} m m+ chains, which interdigitate via multiple weak interactions by Ag–O contacts, are found in the solid structure of 1b. The dissolution of coordination products in coordinating solvents like MeCN or DMSO leads to the decomposition of complexes due to formation of silver-solvent coordination compounds. The coordination products 1–5 are stable in solid state against exposure to the ambient light, whereas solutions of the compounds, especially in DMSO-d6, appeared to be photochemically labile. As revealed by NMR spectroscopic studies, the organic components undergo trans-cis isomerization.
Similar content being viewed by others
References
Metal-Organic Frameworks. Applications from Catalysis to Gas Storage, Ed. by D. Farrusseng (Wiley-VCH, Weinheim, 2011).
Metal-organic frameworks. Design and Application, Ed. by L. R. Macgillivray (Wiley, Hoboken, 2010).
Topics in Current Chemistry, Vol. 293: Functional Metal-Organic Frameworks Gas Storage, Separation and Catalysis Ed. by M. Schröder (Springer, Berlin/Heidelberg, 2010).
Design and Construction of Coordination Polymers, Ed. by M.-C. Hong and L. Chen (Wiley, Hoboken, 2009).
S. R. Batten, S. M. Neville, and D. R. Turner, Coordination Polymers—Design, Analysis and Application (RSC, 2008).
M. Fujita, Y. J. Kwon, S. Washizu, and K. Orgua, J. Am. Chem. Soc. 116, 1151 (1994).
A. J. Blake, N. R. Champness, P. Hubberstey, et al., Coord. Chem. Rev. 183, 117 (1999).
B. Moulton and M. J. Zavorotko, Chem. Rev. 101, 1629 (2001).
M. Eddaoudi, D. B. Moler, H. Li, et al., Acc. Chem. Res. 34, 319 (2001).
G. Férey, Chem. Mater. 13, 3084 (2001).
M. Eddaoudi, J. Kim, N. Rosi, et al., Science 295, 469 (2002).
C. Janiak, Dalton Trans., 2781 (2003).
S. L. James, Chem. Soc. Rev. 32, 276 (2003).
B. Kesanli and W. Lin, Coord. Chem. Rev. 246, 305 (2003).
S. Kitagawa, R. Kitaura, S. Noro, Angew. Chem. Int. Ed. 43, 2334 (2004).
M. J. Rosseinsky, Micropor. Mesopor. Mater. 73, 15 (2004).
R. Dobrawa and F. Würthner, J. Polym. Sci.: A: Polym. Chem., 43, 4981 (2005).
M. Ruben, J.-M. Lehn, and P. Müller, Chem. Soc. Rev. 35, 1056 (2006).
N. R. Champness, Dalton Trans., 877 (2006).
C. J. Kepert, Chem. Commun., 695 (2006).
C.-F. Chow, S. Fujii, and J.-M. Lehn, Angew. Chem. Int. Ed. 46, 5007 (2007).
D. N. Dybtsev, M. P. Yutkin, E. V. Peresypkina, et al., Inorg. Chem. 46, 6843 (2007).
A. D. Burrows, C. G. Frost, M. F. Mahon, and C. Richardson, Angew. Chem. Int. Ed. 47, 8482 (2008).
S. Bureekaew, S. Shimomura, and S. Kitagawa, Sci. Technol. Adv. Mater. 9, 014108 (2008).
G. Ferey, Chem. Soc. Rev. 37, 191 (2008).
J. R. Long and O. M. Yaghi, Chem. Soc. Rev. 38, 1213 (2009).
L. J. Murray, M. Dinca, and J. R. Long, Chem Soc. Rev. 38, 1294 (2009).
Sh. Noro, S. Kitagawa, T. Akutagawa, and T. Nakamura, Progr. Polym. Sci. 34, 240 (2009).
S. Horike, S. Shimomura, and S. Kitagawa, Nat. Chem. 1, 695 (2009).
W. L. Leong and J. J. Vittal, Chem. Rev. 111, 688 (2010).
A. D. Naik, M. M. Dîrtu, A. P. Railliet, et al., Polymers 3, 1750 (2011).
S. Dalai, J. Phys. Sci. 15, 223 (2011).
S. R. Batten, N. R. Champness, X.-M. Chen, et al., Cryst. Eng. Commun. 14, 3001 (2012).
C.-P. Li, C.-S. Liu, and S.-M. Fang, Coord. Chem. Rev. 257, 1282 (2013).
C.-Y. Su, Coord. Chem. Rev. 2013, 257, 1373 (2013).
T. Yamada, K. Otsubo, R. Makiura, and H. Kitagawa, Chem. Soc. Rev. 42, 6655 (2013).
M. L. Foo, R. Matsuda, and S. Kitagawa, Chem. Mater. 26, 310 (2014).
A. N. Khlobystov, A. J. Blake, N. R. Champness, et al., Coord. Chem. Rev. 222, 152 (2001).
S.-L. Zheng, M.-L. Tong, and X.-M. Chen, Coord. Chem. Rev. 246, 185 (2003).
M. N. Kozlova, S. Ferlay, S. E. Solovieva, et al., Dalton Trans., 5126 (2007).
Y.-B. Dong, J.-P. Ma, R.-O. Huang, et al., Dalton Trans., 1472 (2003)
M. Oh, C. L. Stern, and C. A. Mirkin, Inorg. Chem. 44, 2647 (2005).
S. Z. Vatsadze, A. G. Golikov, A. P. Kriven’ko, and N. V. Zyk, Russ. Chem. Rev. 77, 661 (2008).
S. Z. Vatsadze, M. A. Kovalkina, N. V. Sviridenkova, et al., Cryst. Eng. Commun. 6, 112 (2006).
S. Z. Vatsadze, I. A. Vatsadze, M. A. Manaenkova, et al., Russ. Chem. Bull., Int. Ed. 56, 1775 (2007).
A. A. M. Aly, S. Z. Vatsadze, A. V. Chernikov, et al., Polyhedron 26, 3925 (2007).
S. Z. Vatsadze, G. V. Gavrilova, F. S. Zyuz’kevich, et al., Russ. Chem. Bull. 65, 1761 (2006).
S. Vatsadze, M. Al-Anber, W. R. Thiel, et al., J. Solid State Electrochem. 9, 764 (2005).
D. Das and K. Biradha, Inorg. Chem. Frontiers, 2017, doi 10.1039/C7QI00328E
M. W. Hosseini, Acc. Chem. Res. 38, 313 (2005).
K. Biradha and R. Santra, Chem. Soc. Rev. 42, 950 (2013).
R. Santra, M. Garai, D. Mondal, and K. Biradha, Chem. Eur. J. 19, 489 (2013).
R. Santra and K. Biradha, CrystEngComm 13, 3246 (2011).
R. Santra, N. Ghosh, and K. Biradha, New J. Chem. 32, 1673 (2008).
R. Santra and K. Biradha, CrystEngComm, 2008, 10, 1524.
S. Z. Vatsadze, M. A. Manaenkova, N. V. Sviridenkova, et al., Russ. Chem. Bull., Int. Ed., 2006, 55, 1184.
Comprehensive Coordination Chemistry, Ed. by G. V. Wilkinson (Pergamon Books Ltd, 1987) vol. 2, ch. 15.5.
K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Parts A+B, Wiley-VCH, 1997.
C. Pettinari, F. Marchetti, A. Cingoloni, et al., Polyhedron, 1998, 17, 1677.
G. A. Lawrance, Chem. Rev., 1986, 86, 17.
A. W. Addison, T. N. Rao, J. Reedijk, et al., J. Chem. Soc., Dalton Trans., 1984, 1349.
Cambridge Structural Database, ConQuest Version 1.12, CCDC 2009.
G. M. Sheldrick, SHELXTL Version 5.1, An Integrated System for Solving, Refining and Displaying Crystal Structures from Diffraction Data, Siemens Analytical X-ray Instruments, Madison, WI, 1990.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Aly, A.A.M., Vatsadze, S.Z., Walfort, B. et al. Coordination polymers of silver(I) with ditopic cross-conjugated dienone. Russ. J. Inorg. Chem. 62, 1584–1594 (2017). https://doi.org/10.1134/S0036023617120038
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023617120038