Abstract
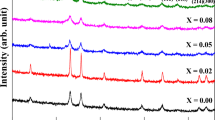
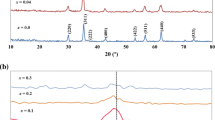
Nanopowders La1–x Zn x FeO3 (nominal degree of doping x nom = 0, 0.05, 0.075, 0.1, 0.15, 0.2, 0.3, and 0.4) were synthesized by the sol–gel method using aqueous ammonia as a precipitator and were then annealed at 950°C for 60 min. From the data of X-ray powder diffraction analysis and local electron probe microanalysis, the maximum actual limit of doping of lanthanum ferrite with zinc was determined: x = 0.072. The dependence of the particle size on the Zn2+ content was found to be nonmonotonic. The magnetic characteristics (specific magnetization J, coercivity H c, and magnetic susceptibility χ) of samples at temperatures of 300 and 100 K in fields of up to 1300 kA/m. It was shown that, with increasing degree of doping, J increases from 0.188 A m2/kg (at x = 0) to 0.245 A m2/kg (at x = 0.072), and χ varies nonmonotonically from 11.5 × 10–6 (at x = 0) to 15.3 × 10–6 (at x = 0.072) (at 300 K). With decreasing measurement temperature to 100 K, the magnetization and susceptibility monotonically increase.
Similar content being viewed by others
References
F. J. Berry, X. Ren, J. R. Gancedo, and J. F. Marco, Hyperfine Interact. 156, 335 (2004).
Q. Zhang and F. Saito, J. Mater. Sci. 36, 2287 (2001).
D. Bayraktar, F. Clemens, S. Diethelm, et al., J. Eur. Ceram. Soc. 27, 2455 (2007).
G. V. Bazuev, N. A. Zaitseva, V. N. Krasil’nikov, and D. G. Kellerman, Russ. J. Inorg. Chem. 48, 170 (2003).
A. F. Guseva, A. Ya. Neiman, and S. S. Nokhrin, Methods for Producing Nanosized Materials (UrGU, Yekaterinburg, 2008) [in Russian].
V. Yu. Kurochkin, A. A. Il’in, and A. P. Il’in, Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tekhnol. 53, 90 (2010).
Nguyen Anh Tien, V. O. Mittova, I. Ya. Mittova, and Dinh Van Tac, Kondens. Sredy Mezhfaznye Granitsy 12, 56 (2010).
Nguyen Anh Tien, M. V. Knurova, Nguyen Thi Mo, et al., Nanosystems: Phys. Chem. Math. 5, 672 (2014).
V. A. Rabinovich and Z. Ya. Khavin, Concise Chemical Handbook (Khimiya, Leningrad, 1991) [in Russian].
K. Mukhopadhyay, A. S. Mahapatra, and P. K. Chakrabarti, J. Magn. Magn. Mater. 329, 133 (2013).
Shuhua Dong, Kejing Xu, and Guishan Tian, J. Mater. Sci. 44, 2548 (2009).
K. Mukhopadhyay, A. S. Mahapatra, and P. K. Chakrabarti, Mater. Lett. 159, 9 (2015).
Irshad Bhat, Shahid Husain, Wasi Khan, and S. I. Patil, Mater. Res. Bull. 48, 4506 (2013).
S. S. Karpova, V. A. Moshnikov, S. V. Myakin, and E. S. Kolovangina, Fiz. Tekh. Poluprovodn. (St. Petersburg) 47, 369 (2013).
Nguyen Anh Tien, I. Ya. Mittova, O. V. Almjasheva, et al., Glass Phys. Chem. 34, 756 (2008).
A. I. Gusev, Nanomaterials, Nanostructures, and Nanotechnologies, (Fizmatlit, Moscow, 2005) [in Russian].
D. Brandon and W. D. Kaplan, Microstructural Characterization of Materials (Wiley, Chichester, 1999).
JCPDC PCPDFWIN: A Windows Retrieval/Display Program for Assessing the ICDD PDF-2 Database (International Centre for Diffraction Data, 1997).
M. V. Knurova, Nguyen Anh Tien, V. O. Mittova, and I. Ya Mittova, Proceedings of the Third International Conference of CIS Countries on Sol–Gel Synthesis and Investigation of Inorganic Compounds, Hybrid Compounds, and Disperse Systems, September 8–12, 2014, Suzdal’, Russia (OAO Izdatel’stvo Ivanovo, Ivanovo, 2014), p. 137 [in Russian].
O. V. Al’myasheva, B. A. Fedorov, A. V. Smirnov, and V. V. Gusarov, Nanosyst.: Phys., Chem., Math. 1, 26 (2010).
R. Zboril, M. Mashlan, and D. Petridis, Chem. Mater. 14, 969 (2002).
K. P. Belov, Magnetostriction Phenomena and Their Technical Applications (Nauka, Moscow, 1987) [in Russian].
V. M. Novotortsev, S. F. Marenkin, L. I. Koroleva, et al., Russ. J. Inorg. Chem. 54, 1350 (2009).
S. F. Marenkin, V. M. Trukhan, I. V. Fedorchenko, et al., Russ. J. Inorg. Chem. 59, 355 (2014).
S. F. Marenkin, A. D. Izotov, I. V. Fedorchenko, and V.M. Novotortsev, Russ. J. Inorg. Chem. 60, 295 (2015).
D. P. Tang, R. Yuan, and Y. Q. Chai, Biotechnol. Lett. 28, 559 (2006).
A. K. Gupta and M. Gupta, Biomater. 26, 3995 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.V. Knurova, I.Ya. Mittova, N.S. Perov, O.V. Al’myasheva, Nguyen Anh Tien, V.O. Mittova, V.V. Bessalova, E.L. Viryutina, 2017, published in Zhurnal Neorganicheskoi Khimii, 2017, Vol. 62, No. 3, pp. 275–282.
Rights and permissions
About this article
Cite this article
Knurova, M.V., Mittova, I.Y., Perov, N.S. et al. Effect of the degree of doping on the size and magnetic properties of nanocrystals La1 – x Zn x FeO3 synthesized by the sol–gel method. Russ. J. Inorg. Chem. 62, 281–287 (2017). https://doi.org/10.1134/S0036023617030081
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023617030081