Abstract
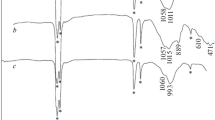
Manganese oxides have been prepared on the surface of carbon fiber by simple methods: coprecipitation of manganese salts of different valence in the presence of fiber as a support or electrodeposition from Mn(II) salt solution on a carbon fiber cathode, in the presence of chitosan including, under oxidation with air oxygen conditions. The obtained samples have been characterized by scanning electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy. Sorption properties of the composites toward As(V) have been studied. The relationships between sorption properties, structure, Mn valence, and manganese oxide surface morphology have been discussed.
Similar content being viewed by others
References
S. Shanmugam and A. Gedanken, J. Phys. Chem. B 110, 24486 (2006).
L.-C. Wang, Q. Liu, X.-S. Huang, et al., Appl. Catal. B: Environ. 88, 204 (2009).
S. Ching, K. S. Krukowska, and S. L. Suib, Inorg. Chim. Acta 294, 123 (1999).
S. A. Kirillov, T. V. Lesnichaya, N. M. Visloguzova, et al., New Carbon Based Materials for Electrochemical Energy Storage Systems: Batteries, Supercapacitors and Fuel Cells (Springer, Dordrecht, 2006).
T. Brousse, M. Toupin, R. Dugas, et al., J. Electrochem. Soc. 153, A2171 (2006).
L.-C. Zang, Z.-H. Liu, H. Lv, et al., J. Phys. Chem. 111, 8418 (2007).
E. Raymundo-Piñero, V. Khomenko, E. Frackowiak, and F. Béguin, J. Electrochem. Soc. 152, A229 (2005).
Y. U. Jeong and A. Manthiram, J. Electrochem. Soc. 149, A1419 (2002).
S.-C. Pang, M. A. Anderson, and T. W. Chapman, J. Electrochem. Soc. 147, 444 (2000).
M. Nakayama, T. Kanaya, and R. Inoue, Electrochem. Commun. 9, 1154 (2007).
J. N. Broughton and M. J. Brett, Electrochim. Acta 50, 4814 (2005).
H. Kanoh, W. Tang, Y. Makita, and K. Ooi, Langmuir 13, 6845 (1997).
P. Stouff and J. Boulégue, Am. Mineral. 73, 1162 (1988).
D. C. Golden, J. B. Dixon, and C. C. Chen, Clay Clay Miner. 34, 511 (1986).
Y.-F. Shen, S. L. Suib, and C.-L. O’ Young, J. Am. Chem. Soc. 116, 11020 (1994).
J. Luo and S. L. Suib, Chem. Commun. 1031 (1997).
M. Nakayama, S. Konishi, H. Tagashira, and K. Ogura, Langmuir 21, 354 (2005).
M. Nakayama and H. Tagashira, Langmuir 22, 3864 (2006).
J.-J. Xu, X.-L. Luo, Y. Du, and H.-Y. Chen, Electrochem. Commun. 6, 1169 (2004).
N. Nagarajan, M. Cheong, and I. Zhitomirsky, Mater. Chem. Phys. 103, 47 (2007).
A. Dyer, M. Pillinger, R. Hajula, and S. Amin, J. Mater. Chem. 10, 1867 (2000).
A. Dyer, M. Pillinger, J. Newton, et al., Chem. Mater. 12, 3897 (2000).
E. V. Saenko, Extended Abstract of Candidate’s Dissertation in Chemistry (Perm, 2007).
G. V. Leont’eva, L. G. Chirkova, and V. V. Vol’khin, Zh. Prikl. Khim. 53, 1229 (1980).
G. V. Leont’eva, Zh. Prikl. Khim. 70, 1615 (1997).
S. Ouvrard, M.-O. Simonnot, and M. Sardin, Ind. Eng. Chem. Res. 41, 2785 (2002).
K. A. Kovalenko, G. R. Bochkarev, and G. I. Pushkareva, Voda: Khim. Ekol., No. 10, 80 (2013).
N. Ajith, A. A. Dalvi, K. K. Swain, et al., J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng. 48, 422 (2013).
B. A. Manning, S. E. Rendorf, B. Bostick, and D. L. Suarez, Environ. Sci. Technol. 36, 976 (2002).
B. J. Lafferty, M. Ginder-Vogel, and D. L. Sparks, Environ. Sci. Technol. 44, 8460 (2010).
B. J. Lafferty, M. Ginder-Vogel, M. Zhu, et al., Environ. Sci. Technol. 44, 8467 (2010).
B. J. Lafferty, M. Ginder-Vogel, and D. L. Sparks, Environ. Sci. Technol. 45, 9218 (2011).
K. Babaeivelni, A. P. Khodadoust, and D. Bogdan, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng. 49, 1462 (2014).
D. Ocinski, I. Jacukowicz-Sobala, and E. Kociolek-Balawejder, J. Appl. Polym. Sci. 131, 39489 (2014), doi 10.1002/APP.39489
C. Tournassat, L. Charlet, D. Bosbach, and A. Manceau, Environ. Sci. Technol. 36, 493 (2002).
M. Villalobos, I. N. Escobar-Quiroz, and C. Salazar-Camacho, Geochim. Cosmochim. Acta 125, 564 (2014).
L. A. Zemskova, I. V. Sheveleva, N. N. Barinov, et al., Russ. J. Appl. Chem. 81, 1187 (2008).
L. A. Zemskova, A. V. Voyt, N. N. Barinov, and T. A. Kaydalova, Glass Phys. Chem. 40, 1 (2014).
G. H. A. Therese and P. V. Kamath, Chem. Mater. 12, 1195 (2000).
Yu. Yu. Lur’e, Analytical Chemistry of Industrial Wastes (Khimiya, Moscow, 1984) [in Russian].
R. T. Cygan, J. E. Post, P. J. Heaney, and J. D. Kubiski, Am. Mineral. 97, 1505 (2012).
M. Chigane and M. Ishikawa, J. Electrochem. Soc. 147, 2246 (2000).
J. M. Cerato, M. F. Hochella, W. R. Knocke, et al., Environ. Sci. Thechnol. 44, 5881 (2010).
M. Zhu, K. W. Paul, J. D. Kubiski, and D. L. Sparks, Environ. Sci. Technol. 43, 6655 (2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © L.A. Zemskova, A.V. Voit, N.N. Barinov, Yu.M. Nikolenko, D.Kh. Shlyk, 2016, published in Zhurnal Neorganicheskoi Khimii, 2016, Vol. 61, No. 12, pp. 1628–1634.
Rights and permissions
About this article
Cite this article
Zemskova, L.A., Voit, A.V., Barinov, N.N. et al. Composite sorbents based on synthetic manganese oxide and carbon fiber. Russ. J. Inorg. Chem. 61, 1567–1572 (2016). https://doi.org/10.1134/S0036023616120226
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023616120226